Nanomedicine and Nanoscience Technology: Open Access

Research - (2021) Volume 1, Issue 1
Bismuth Oxide Nanoparticle: The Potential of Apoptotic and Genetic Damage on MDBK Cell Line
2Department of Biology, Mersin University, Mersin, Turkey
3Department of Advanced Technology, Mersin University, Mersin, Turkey
Received Date: Oct 04, 2021 / Accepted Date: Nov 02, 2021 / Published Date: Nov 10, 2021
Abstract
Nowadays, nanoparticles have been used in almost all areas of life thanks to advances in nano scale technology. Among the available nanoparticles, Bismuth oxide nanoparticle is widely used in many products in terms of its properties such as antibacterial, antitumor and cytotoxic activity and antifungal. In this study, we aimed to determine the in vitro genetic damage potential and apoptotic effects of bismuth oxide nanoparticle having 90-210 nm size (~191,2 nm) range in MDBK cell line cultures. In this study; single cell gel electrophoresis (comet) method and flow cytometry method were used to determine genotoxic and apoptotic effect. The MDBK (Madin Darby Bovine Kidney) cells were treated with bismuth oxide at three concentrations of 30 µg/ml, 60 µg/ml and 90 µg/ml. No significant difference was found between the negative control group for GDI and DCP parameter (p>0.05). When 30 µg/ml concentration was considered, there was a significant difference between negative control and other groups for early and late apoptotic, necrotic cell and number of live cells (p<0.05). Considering the 60 µg/ml and 90 µg/ml concentration, there was a statistically significant difference between the other groups in terms of early apoptotic, necrotic and live cell counts (p<0.05). There was a significant difference between positive control and negative control in terms of early and late apoptotic, necrotic cell and number of live cells (p<0.05).
Keywords
Genetic damage; Bismuth oxide nanoparticle; Flow cytometry; Cell line
Abbreviations
MDBK: Madin Darby Bovine Kidney; NMA: Normal Melting Agarose; PBS: Phosphate Buffer Saline
Introduction
Nanotechnology is a complementary discipline that explores and manipulates physical substances on the nanometers scale, representing a unique combination of natural, mathematical, computer and material sciences [1]. With the development of nanotechnology nano-sized materials such as nanocrystals, nanoparticles and nanotubes can be produced. The NPs, which have gained great importance in recent years, constitute the main basis of nanotechnology.
Nowadays, the use of nanoparticles in many areas of nanotechnology needs to be used. Therefore, it is necessary to examine the potential effects of nanoparticles to protect human and environmental health [2]. Despite the advantages and advances of nanoparticles to the world, there has been serious debate about the dissemination of nanotechnology, and thus the potential risks associated with its disadvantages in the world recently are expected [3].
Nanoparticles can be classified in different ways according to their properties. Metal nanoparticles are prepared from metal precursors [4]. Bismuth, the chemical symbol "B" a semi-metal. Bi2O3 is one of the important metal oxides and provides a suitable environment for biomolecule adsorption. Bi2O3 nanoparticles have better adsorption properties than regular sized particles due to their greater advantages and new properties (higher specific surface, more surface free energy and good electrochemical stability, etc.) [5]. In a study, Abudayyak et al. have investigated the toxic effects in order to evaluate the toxicity of Bi2O3 nanoparticles in mammalian cells (HepG2 hepatocarcinoma cell), kidney (NRK-52E renal epithelial cell), intestine (Caco-2 colorectal adenocarcinoma cell) and lung (A549 lung carcinoma cell) [6]. Recent studies have shown that metal nanoparticles have cytotoxic and genotoxic effects on humans [7]. Bismuth oxide is seen as the least toxic heavy metal for humans and is widely used in medical applications for good antibacterial properties. Bismuth oxide nanoparticles have been used in different sizes in vivo and in vitro experiments [5,6].
One of the branches of toxicology, genetic toxicology, is a discipline that examines the changes in DNA molecules of cells during the normal biological functioning of the organism and plays an important role in the evaluation of genetic damage caused by different agents. Mutagenic agents or mutagens on the DNA molecule show their effects directly or indirectly by binding to proteins synthesized according to genomic information. The key molecules and pathways involved in DNA damage are tissue damage, aging, cancer, infertility and some genetic and multi factorial diseases [8].
Genotoxic effect can be measured by many different techniques and methods. The single cell gel electrophoresis, comet assay, method based on the principle of determination of fractures on the DNA molecule at the gene level has found widespread use in the determination of many DNA damage and repair, and in the bio monitoring and exposure studies. Comet method is a simple, sensitive and rapid method because it has become a useful and preferred method in genetic toxicology studies [9-11].
Each cell has a certain life span. There is a controlled balance between cell death and cell proliferation. Apoptosis, which is one of the cell death types, refers to the petal that is separated from the fallen leaf or flower in Greek. Cell death due to DNA damage is performed by apoptosis, necrosis and autophagy. Apoptosis, which is one of these different forms of cell inactivation, is the main pathway after DNA damage [12]. Flow cytometry is the measurement of the characteristics of cells or particles in a flowing fluid. Cells or particles suspended in flow cytometry are passed through a compartment illuminated by laser light; when the cells pass through the light, the signals they give are analyzed. The source of the signals formed may be the physical properties of the cell, such as size and granularity; there may also be various fluorochromes which bind to the cell. Thus information can be collected about various features such as immunophenotype of the cell or particle, DNA content, enzyme activities, cell membrane potential, viability [13,14].
Some in vivo studies have shown that different NPs, including ZnO, can be retained in the kidney [15]. Kidney cells were shown to be reliable for detecting a NP dose-response. In our study, we used the Madin-Darby Canine Kidney (MDCK) as an in vitro model of renal epithelial cells and aimed to assess the possible cellular toxicity/apoptotic and genotoxicity mechanism of Bi2O3 nanoparticles and the correlation between flow cytometry parameters and comet assayparameters. The study was designed to investigate their cytotoxicity, genoto-oxidative damage and apoptotic potential in MDBK cells.
Materials and Methods
Chemicals
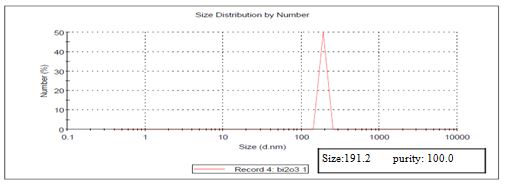
Bismuth oxide (Bi2O3) (High purity, 99.95%, CAS Number 1314-23-4) was obtained from US Research Nanomaterials, Inc. According to the manufacturer, Bismuth oxide Nanopowder, Crystal Phases: monoclinic, APS: 90-210 nm, Color: yellow, Morphology: shape near spherical True Density: 5.89 g/cm3. A stock solution of Bi2O3 (500 μl) using steriled distilled water was prepared and stored at 4°C in darkness. DMEM and fetal Calf serum were purchased from Sigma. Other chemicals or solvents used in this study were of cell culture, HPLC, or analytical grade. The size of nanoparticle was measured using nanosizer at Advanced Technology, Education, Research and Application Center in Mersin University. The size of nanoparticle was 191,2 nm (Figure 1).
FIG.1. Results of Size Measurement of Bi2O3 Nanoparticle.
The selection of dose and the size of nanoparticle
The studies performed with Bi2O3 reported that the concentration of 30 µg/ml showed the genotoxic and cytotoxix effect in many cell lines [16,17]. Therefore; in this study, particle size and concentration were selected as ~191,2 nm and 30 μg/ml, 60 μg/ml and 90 μg/ml, respectively.
Cell culture design
MDBK cells were grown in DMEM media with 10% fetal bovine serum, 1%antibiotics (100 μg/ml penicillin and 100 μg/ml streptomycin) and sodium pyruvate. Cells were incubated in 5% CO2 incubator at 37°C. Cell media was refreshed once in every 7 days until they reach confluency (80%) to be used in the experiments.
Bi2O3 and H2O2 treatment of MDBK cells
MDBK cells were put in 4 × 105 cells/well concentration in 1 ml fresh complete DMEM as described above into 12-well plates, then they were rested overnight in 37°C 5% CO2 incubator. We tested 30, 60 and 90 μg/ml of 20 μm filter sterilized Bi2O3's effect on MDBK cells. 10m M of sterile H2O2 was put into 1 mL media of overnight rested cells in positive control wells for COMET assay. Cells were treated with the Bi2O3 and H2O2 for 72 h in 37°C at incubator with 5% CO2. Afterwards cells were collected into eppendorf tubes and centrifuged at 2000 rpm to get rid of supernatants, then cells were re-suspended in PBS and this washing step was repeated 3 times. After getting rid of the last supernatant phase the cells were counted and 100.000 cells were used for COMET assays and 100.000 cells of the each condition were stained with Annexin V and PI dyes to analyze the apoptotic levels via flow cytometry. All experimental conditions were tested as a quadruple.
Comet assay/genoto-oxidative damage
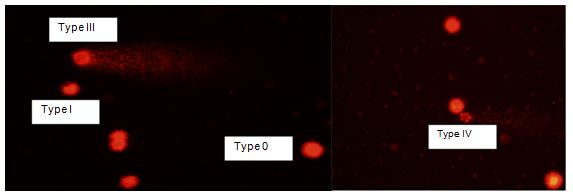
Comet assay was performed under alkaline conditions according to method of Singh et al. with slight modifications [18]. Slides were coated by a thin layer of 0.5% Normal Melting Agarose (NMA) dissolved in Ca2+ and Mg2+ free Phosphate Buffer Saline (PBS)at about 50°C. Eppendorf tubes were placed in water bath at 35°C and then 100 μl of MDBK cell suspension was diluted with 1 ml of PBS in eppendorf tube. Then 100 μl of mixtures were mixed with 100 μl of LMA (0.5%). 100 μl of this mixture was spreaded on NMA-coated slides using micropipette and immediately was covered with cover slip. Slides were conserved in fridge at +4 °C for 20 min. The cover slips were slowly removed from top of slides. Then slides were placed in chalets including lysis solution and conserved for 1 h in refrigerator in dark. Slides were placed on a horizontal gel electrophoresis unit filled with fresh electrophoretic buffer (300 mM NaOH + 1m M EDTA) to allow DNA unwinding before electrophoresis. Electrophoresis was conducted at 20 °C using 25 V and 300 mA for 25 min. The above steps should be carried out in dark to prevent DNA damage. After electrophoresis, slides were placed in neutralizing buffer (pH=7.5) for 15 min. Then slides were placed in chilled ethanol for 10 min. After staining procedur with ethidium bromide (0.1 mg/ml, 1:4) and the slides were examined with a fluorescent microscope (BX51, Olympus, Japan). 100 cells were considered for microscopic evaluation.100 cells were counted from each concentration and the counted cells were classified in five groups according to their damage level: Type 0, Type 1, Type 2, Type 3 and Type 4.
Two parameters were evaluated in comet assay analyses;
- Genetic damage index: Damaged cell percentage, in following formula; Genetic damage index: 0xType 0+1xType I+2xType II+3xType III+4xType IV
- Damaged cell percentage: TypeII+TypeIII+TypeIV Comet assay scores show levels of UD (undamaged, 0), Type 1 (lowdamaged), Type II (moderate damaged), Type III (high damaged), and Type IV (ultra high damaged).
Flow cytometry method/apoptosis assay
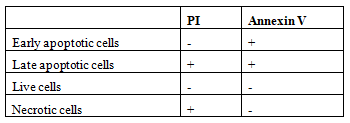
To determine cellular apoptosis or necrosis, Annexin V-FITC apoptosis detection kit with PI was used. The kit is based on observation of the translocation of the membrane phosphatidylserine from the inner side of plasma membrane to cell outer-surface, which can be easily detected by staining with a fluorescent dye Annexin V, a protein that has a high affinity for phosphatidylserine, conjugated to FITC. In flow cytometry analyses, late and early apoptotic cell, live cell and necrotic cell were counted. The cells are grouped according to their staining properties in the following Table 1. Approximately 4 × 105 cells were passed through flow cytometry to determine the amount of drift of the MDBK cells exposed to bismuth oxide nanoparticles to the apoptotic process.
Table 1. Cell stainingproperties.
Statistical analysis
The normality control for all parameters was performed with the Shapiro Wilk’s test. STATISTICA 13.0 analysis program was used to evaluate the data obtained as a result of the experimental protocols. The concentrations applied to determine whether there is a statistically significant difference between the results were compared between both the positive control values and the negative control values. The mean of the data obtained from the experimental protocols was used for all analyzes. The difference between the groups was analyzed by Kruskal Wallis program. When evaluating, p value (confidence interval) was taken as 0.05. Pearson correlation analysis was applied to determine the relationship between Comet and flow cytometry parameters.
Results
In the present study, genotoxicity measurements of the preparations obtained from the MDBK cells exposed to three concentrations of Bi2O3 nanoparticle (30 µg/ml, 60 µg/ ml and 90 µg/ml) were determined by Comet test method. Cell viability was determined by flow cytometry analysis.
Comet analysis results
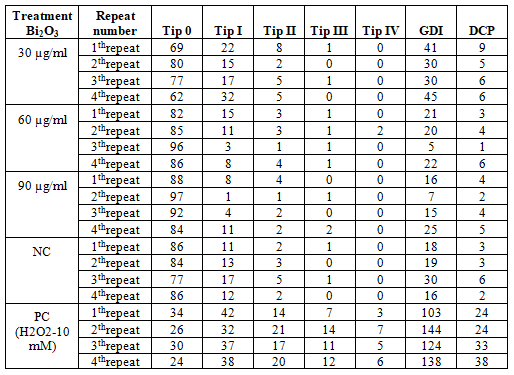
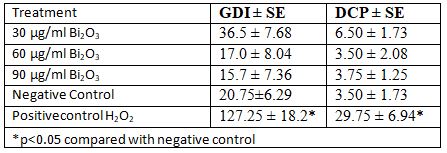
Results of comet analysis obtained from MDBK cell line are shown in Table 2. The images obtained under the fluorescence microscope are shown in Figure 2. An increase in the concentration of GDI and DCP was observed in parallel with the increase in concentration (Figure 3). No significant difference was found between the negative control group and the 30 µg/ml and 60 µg/ml and 90 µg/ml concentration groups in terms of GDI parameter. No significant difference was found between the negative control group and the concentration group of 30 µg/ml and 60 µg/ml and 90µg/ml for the DCP parameter (Table3).
FIG.2. Comet views in ethidium bromide-stained MDBK Cells.
FIG.3. Frequency of GDI and DCP in relation to Bi2O3 nanoparticle concentrations in MDBK cells.
Table 2. Results of Comet Assay Analysis in MDBK cells treated with Bi2O3 nanoparticle (~191.2 nm)
Table 3. Statistical results in MDBK cell treated with Bi2O3 nanoparticle (~191.2 nm) in Genetic damage Index and Damaged cell percent in Comet assay
Flow cytometry analysis results
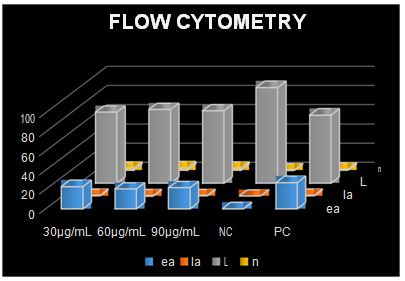
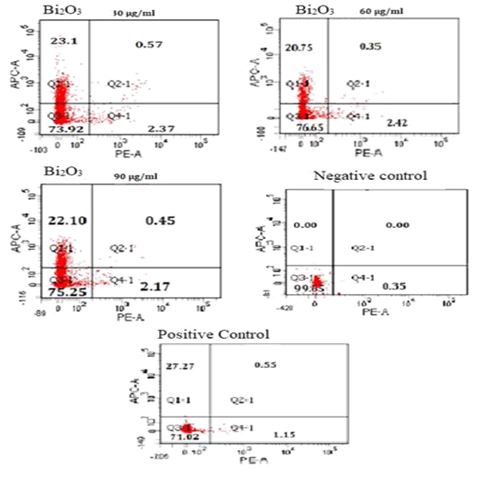
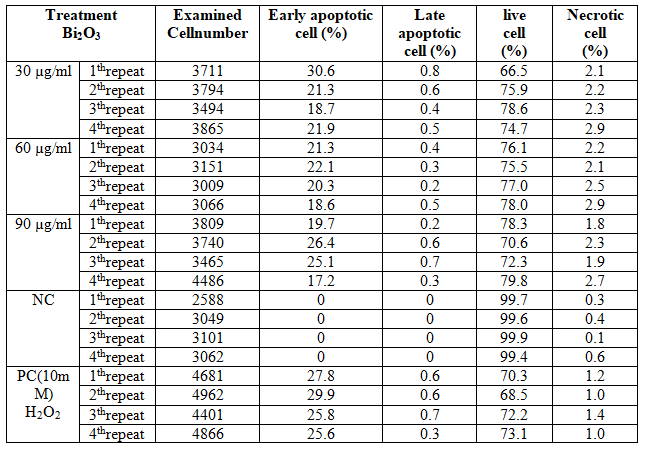
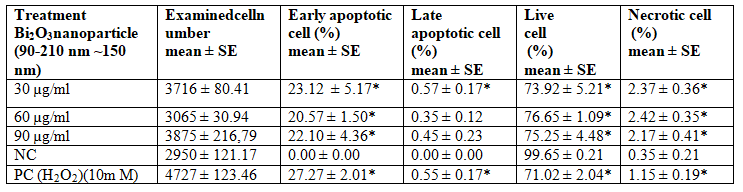
The data obtained as a result of flow cytometry applied to MDBK cells are presented in Table 4. The results of statistical analysis of flow cytometry are given in Table 5. No increase in early apoptotic, late apoptotic, or necrotic cell numbers was observed in parallel with the increase in concentration (Figures 4 and 5). When 30 µg/ml concentration was considered, there was a significant difference between negative control and other groups for early and late apoptotic, necrotic cell and number of live cells (p<0.05). Considering the 60 µg/ml and 90 µg/ml concentration, there was a statistically significant difference between the other groups in terms of early apoptotic, necrotic and live cell counts (p<0.05). There was a significant difference between positive control and negative control in terms of early and late apoptotic, necrotic cell and number of live cells (p <0.05). Table 6 represents results of correlation between comet analysis and flow cytometry data.
FIG.4. Frequency of early apoptotic, late apoptotic, live and necrotic cells in relation to Bi2O3 nanoparticle concentrations in MDBK cells. ea: Early apoptotic, la: Late apoptotic, L: Live, n: Necrotic.
FIG.5. The cells exposed to different Bi2O3 nanoparticle concentrations for 72 h. Cell apoptosis rate after Annexin V-FITC/PI staining was detected by flow cytometry.
Table 4: Flow cytometry results in MDBK cells treated with Bi2O3 nanoparticle (~191.2 nm)
Table 5: Statistical results in MDBK cell treated with Bi2O3 nanoparticle (~191.2 nm) in flow cytometry.
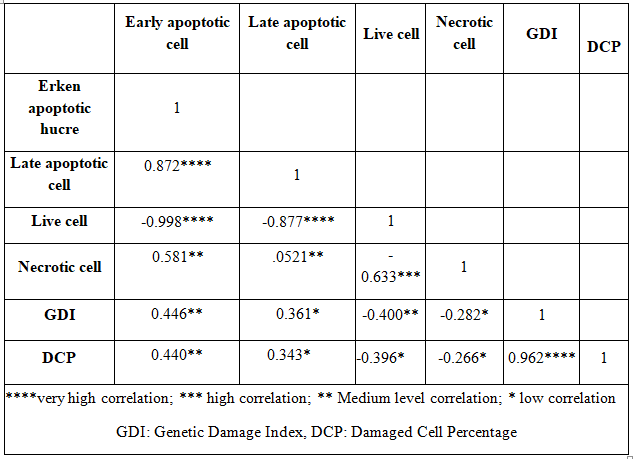
Table 6. Results of Pearson correlation analysis between comet assay parameters and flow cytometry data.
Discussion
In the present study, genotoxicity measurements of the preparations obtained from the MDBK cells exposed to three concentrations of Bi2O3 nanoparticle (30 µg/ml, 60 µg/ml and 90 µg/ml) was determined by Comet test method. Two parameters, GDI and DCP, were evaluated in comet assay method. Cell viability was determined by flow cytometry. In the flow cytometry method, the cells were evaluated according to their early and late apoptotic, viable and necrotic properties. Bi2O3 (~191.2 nm) nanoparticle showed an increase in GDI and DCP values onMDBK cells, but this increase isn’t significant statistically. Based on flow cytometry analysis, there were toxic effects on MDBK cells by Bi2O3 nanoparticle and this effect was statistically significant. Moreover, this effect was more robust at higher concentrations of the nanoparticle. Flow cytometry results showed an increase in early apoptotic, late apoptotic and necrotic cell counts at all concentrations. A high correlation was found between the Comet analysis parameters(r=0.962).There was a medium/low correlation between Comet and flow cytometry analysis parameters(r=0.446, 0.440, 0.361-0.2820.343-0.266)
Controversial results have been reported in data on genetic damage and cell viability in in-vivo and in vitro studies with different cell types in which the effects of different types, sizes and concentrations of prepared nanoparticles are examined.
It has been reported that some bismuth oxide compounds exhibit antibacterial activity and have antimicrobial, antifungal activity. In this area, in the study performed by Hernandez-Delgadillo et al. the bactericidal activity of Bismuth colloidal nanoparticles has been tested with Streptococcus mutans and it is shown to inhibit the growth of Streptococcus mutans [19]. The fungicidal activity of bismuth oxide nanoparticles against Candida albicans was investigated and their antibiotic abilities were analyzed. Bismuth oxide nanoparticles of 77 nm size have been reported to increase C. albicans growth (85% reduction in colony size) and to inhibit biofilm formation and to show antimicrobial activity. These results have been reported to be more effective than those obtained with the most effective oral antiseptic and commercial antifungal agents, chlorhexidine, nystatin and terbinafine. These results suggest that bismuth oxide colloidal nanoparticles may be a very interesting candidate as a fungicidal agent for incorporation into an oral antiseptic. It is compatible with our study in terms of toxic effect when compared on a cellular basis. Abudayyak et al. evaluated the toxic effects of Bi2O3 nanoparticles in four different cell types; liver (HepG2 hepatocarcinoma cell), kidney (NRK-52E renal epithelial cell), intestine (Caco-2 colorectal adenocarcinoma cell) and lung (A549 lung carcinoma cell) [6]. It is determined that Bi2O3 nanoparticles (~149.1 nm) were easily obtained by all cells and showed cytotoxic / genotoxic effects.Host cell death pathways were apoptosis in HepG2 and NRK-52E cells and Bi2O3 nanoparticles were observed to have necrotic effect in A549 and Caco-2 cells. In also our study, Bi2O3 nanoparticule has necrotic effect on MDBK cells. When two studies are evaluated, they support each other.In addition, the increase in 8-hydroxy deoxyguanine (8-OHdG) levels was reported by Ozcan et al. as a marker of DNA damage in response to oxidative stress [20]. Increased levels of 8-hydroxy deoxyguanine (8-OHdG) are important to demonstrate damage to DNA. The reactive oxygen species change by influencing the bases in DNA and the products formed by the changing bases include more than 20 samples, such as thymine peroxyl radicals, hydroxy hydroperoxide, thymine glycol, 5-hydroxymethylthuracil, 5-formyluracil and 5-hydroxy-5-methylhydantoin mentioned. 8-OHdG (8-hydroxy deoxyguanosine) is the most well-known of these damaged bases. Therefore, 8-OHdG form oxidative modified DNA is used to determine the amount of DNA damage. Hydroxyl radicals (OH) interact in the 8th position in the guanine molecule to cause oxidation. The oxidative damage of the modified DNA results in 8-OHdG (8-hydroxy deoxyguanosine). In addition, Cu+2 ions bind with high affinity especially to guanine bases of DNA and interact with H2O2 and have been reported to contribute to DNA damage.
Ahamed et al.investigated the dose-dependent cytotoxicity and apoptosis response of the Bi2O3 nanoparticles on the human breast cancer cell line (MCF-7) and reported that the potential cytotoxicity mechanisms of the Bi2O3 nanoparticles were formed by oxidative stress [21]. They showed that Bi2O3 nanoparticles reduce cell viability and induce membrane damage in a dose range of 50-300 μg / ml depending on the dose. In a physicochemical study, they showed that the Bi2O3 nanoparticles had a crystalline structure and a spherical form with an average structure of 97nm, and showed that Bi2O3 nanoparticles reduced cell viability in toxicity studies and induced membrane damage in a concentration range of 50-300 μg/ml. In addition, exposure of the MCF-7 cells to the Bi2O3 nanoparticles revealed that the expression level of Bcl-2, Bax and caspase-3 genes resulted in apoptotic response. In our study, the presence of medium and low correlation between the flow cytomere and comet analysis parameters showed that the two analyzes were supported by each other. In addition, in the previous studies, it was reported that the increase in the Tip4 comet parameter can be taken as an indicator of apoptotic response, although the increase in comet analysis is not statistically significant compared to the negative control in DNA damage parameters [22,23]. The increase in apoptotic gene expression levels in MCF7 cells coincides with the Comet data of our study. In another study, Akbarzadeh et al Investigated the effects of bismuth oxide folate and 5-aminolevulinic acid (5-ALA) on oral epidermoid carcinoma cells (CB) and lung cancer (A549) cell lines [24]. In this study, the cytotoxic effect of bismuth oxide nanoparticle on cells was evaluated both alone and in combination with folate (5-ALA). KB and A549 cells were incubated with Bi2O3 nanoparticles and folate-5-ALA-conjugated Bi2O3 nanoparticles at 10, 20, 50 and 100 µg/ml concentrations. Cytotoxic effect was tested using MTT method. In addition, nanoparticle-induced apoptosis in the treated cells was obtained using Caspases-3 activity assay and flow cytometry analysis. Bi2O3 nanoparticles with an average of 19.2 ± 6.5 nm successfully synthesized were then conjugated with 5-ALA and folate. Bi2O3 or folate conjugated nanoparticles were easily get into by the cells in a concentration-dependent manner and determined to exhibit cytotoxic effects. Significant cell death was recorded at concentrations of more than 50 µg/ml for both compounds. The prepared nanoparticles showed low cytotoxicity at low incubation times. However, increased concentrations of nanoparticles and cytotoxicity were reported to be increased. In our study, in the flow cytometry analysis, an increase in the parameters of early and necrotic cell counts showed that the cause of cell death is supported by the data. Because the particle size of the nanoparticle used is different, it is not appropriate to compare two studies in terms of particle size, but it is similar in terms of the response of KB and A549 cells to nanoparticles. Liu et al. investigated human toxicity of the bismuth nanoparticle in human embryonic kidney cells (HEK293) in a study in which they were taken into the cell by renal cells, induced autophagy and increased the amount of LC3II protein, and the bismuth nanoparticle had toxic effects on embryonic kidney cells [25]. It has a similarity with the toxicity effect of our study. Shakibaie et al. reported that the bismuth nanoparticles of bacterial origin between 20 and 120 nm have cytotoxic potential in cancer cells such as A549, MCF-7 and 3T3 normal fibroblast cells [26]. Chemical structures of bismuth nanoparticles and organic and inorganic compounds on their surfaces have been shown to play an effective role in their cytotoxicity. The toxic effect of bismuth nanoparticle was consistent with flow cytometry results in our study.Reus et al.(2018) investigated the effects of 250 nm bismuth nanoparticles on BALB/c3T3 cells synthesized by LASIS method. They reported that the morphological structure of the cell was disrupted by the introduction of nanoparticles into the cells and cell death was related to the apoptotic process using the TUNEL method. Because the method we use in our study is a method based on the determination of apoptotic cells, the increase in both early and late apoptotic cells in flow cytometry analysis supports the results of the study conducted by Reus et al [17]. In another study, Bogusz et al. evaluated the toxic effects of Bi(OH)3 and α-Bi2O3 nanoparticleson malignant gliosarcoma 9L and MCF-7human breast cancer cells. Clonogenic assay displaysa mortality rate of over 90% in 9L andMCF-7 cells for a concentration of 50 μg/mL after incubation for 24 hrs for Bi(OH)3 and α-Bi2O3 nanoparticles [16]. In contrast to, at the same concentration, the nano materials exhibit no remarkable mortality in normal Madin-Darby canine kidney cells.
References
- Koopmans RJ, Aggeli A. Nanobiotechnology quo vadis? Current Opinion in Microbiology. 2010; 13(3): 327.
- Abeer S. Future medicine: Nanomedicine. JIMSA. 2012; 25:187-192.
- Khan A. Ethical and social implications of nanotechnology. Science Proceedings. 2015; 57.
- Capek I. Noble metal nanoparticles: Preparation, Composite Nanostructures, Biodecoration and Collective Properties. 2017.
- Liman R. Genotoxic effects of Bismuth (III) oxide nanoparticles by Allium and Comet assay. Chemosphere. 2013; 93(2):269-273.
- Abudayyak M, Oztas E, Arici M, et al. Investigation of the toxicity of bismuth oxide nanoparticles in various cell lines. Chemosphere. 2017; 169:117-123.
- Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008; 14(5):1310-1316.
- Ehrenberg L, Brookes P, Druckrey H, Lagerlof B, et al. The relation of cancer induction and genetic damage. In evaluation of Genetic Risks of Environmental Chemicals, Report of Group 3, Ambio Special Report No. 3, Royal Swedish Academy of Sciences, Universitets for laget, 1973.
- Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000; 35: 206-221.
- Collins AR, Oscoz AA, Brunborg G, et al. The comet assay topical issues. Mutagenesis. 2008; 23:143-151.
- Çelik A, Eke D, Ekinci SY, et al. The protective role of curcumin on perfluorooctane sulphonate-induced genotoxicity: Single cell gel electrophoresis and micronucleus test. Food Chem Toxicol. 2013; 53:249-255.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. British Journal of Cancer. 1972; 26(4):239.
- Toduka Y, Toyooka T, Ibuki Y. Flow cytometric evaluation of nanoparticles using side-scattered light and reactive oxygen species-mediated fluorescence-correlation with genotoxicity. Environ Sci Technol. 2012; 46:7629-7636.
- Pozarowski P, Grabarek J, Darzynkiewicz Z. Flow cytometry of apoptosis. Cur Proto Cell Biol. 2003; 21(1): 18-28.
- Li CH, Shen CC, Cheng YW, et al. Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxidenanoparticles in mice. Nanotoxicol. 2012; 6:746-756.
- Bogusz K, Tehei M, Cardillo D, et al. High toxicity of Bi(OH)3 and -Bi2O3 nanoparticles towards malignant 9L and MCF-7 cells. Materials Science & Engineering C. 2018; 93:958-967.
- Reus TL, Machado TN, Bezerra AG, et al. Dose-dependent cytotoxicity of bismuth nanoparticles produced by LASiS in a reference mammalian cell line BALB/c3T3. Toxicology in Vitro. 2018; 53:99-106.
- Singh NP, McCoy MT, Tice RR, et al. Simple technique for quantitation of low levels of DNA damage in individuals cells. Exp Cell Res. 1988; 175:184-191.
- Hernandez-Delgadillo R, Velasco-Arias D, Martinez-Sanmiguel JJ, et al. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int J Nanomed. 2013; 8:1645.
- Ozcan O, Erdal H, Çakırca G, et al. Oxidative stress and its impacts on intracellular lipids, proteins and DNA. J Clin Exp Invest. 2015; 6(3):331-336.
- Ahamed M, Akhtar MJ, Khan MM, et al. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere. 2019; 216:823-831.
- Eke D, Çelik A, Yilmaz MB, et al. Apoptotic gene expression profiles and DNA damage levels in rat liver treated with perfluorooctane sulfonate and protective role of curcumin. Int J Biol Macromol, 2017; 104:515-520.
- Hao H, Nancai Y, Wen S, et al. The single-cell gel electrophoresis assay to determine apoptosis induced by siRNA in Colo 320 cells. African J Biotech. 2009; 8(16):3731-3733.
- Akbarzadeh F, Khoshgard K, Hosseinzadeh L, et al. Investigating the Cytotoxicity of Folate-Conjugated Bismuth Oxide Nanoparticles on KB and A549 Cell Lines. Adv Pharm Bulletin, 2018: 8(4); 627-635
- Liu Y, Zhuang J, Zhang X, et al. Autophagy associated cytotoxicity and cellular uptake mechanisms of bismuth nanoparticles in human kidney cells. Toxicology letters. 2017: 275; 39-48.
- Shakibaie M, Amiri-Moghadam P, Ghazanfari M, et al. Cytotoxic and antioxidant activity of the biogenic bismuth nanoparticles produced by Delftia sp. SFG. Mat Res Bulletin, 2018; 104:155-163.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.