Nanomedicine and Nanoscience Technology: Open Access

Research Article - (2021) Volume 1, Issue 1
Circulating Exosomal microRNAs as Prognostic Biomarkers in Cholangicarcinoma: A Systematic Review
2College of Medicine and Public Health, Center for Excellence in Biomedical Science and Engineering, Ubon Ratchathani, Thailand
3Department of Medical Technology and Sciences, International University of Health and Welfare, Ohkawa, Japan
Received Date: Oct 06, 2021 / Accepted Date: Nov 12, 2021 / Published Date: Nov 20, 2021
Abstract
Introduction: Cholangiocarcinoma (CCA) is a cancer that arises from the cells within the bile ducts; both inside and outside the liver. Exosomal microRNAs (miRNAs) have been attracting major interest as potential biomarker in cancer. Our aim is to do a systematic review on exosomal miRNAs serve as candidate clinical biomarkers in CCA.
Materials and methods: Electronic databases (Medline, EMBASE, CINAHL and Cochrane data bases). We searched to identify publication all over the world related cancer and miRNA. PRISMA guidelines were followed. Selection was based on the design (Cholangiocarcinoma, invasive cholangiocarcinoma, intra-hepatic, extra-hepatic, liver cancer, microRNA, circulating miRNA, non-coding RNA, exosomal miRNA and exosomal vesicle), 27 papers were selected all over the world. CCA, target antigens, methodologies used for detection and miRNA expression were identified and summarized.
Results: A total of 855 articles were searched, 333 duplicates removed and remain 522, 491 excluded after screened the titles and abstracts; and remain 31, 4 full texts articles were excluded with reason, and27 identifications were included in the studies. Assay method was used to diagnose such as molecular Real Time-qPCR technique.
Conclusion: The findings of this systematic review showed that mircroRNA functions as a tumor suppressor or a promoter in cholangiocarcinoma.
Keywords
Cholangiocarcinoma; Intra-hepatic; Extra-hepatic; microRNA; Medline; EMBAS; CINAHL; Cochrane; Systematic review
Introduction
Cholangiocarcinoma (CCA) is an aggressive and fatal malignancy in the intra- and extra-hepatic biliary tract with increase in incidence and dismal prognosis according to the statistics, the incidence and mortality of CCA is raising worldwide [1,2]. This type of cancer frequently diagnosed as the second leading cause of cancer-related mortality worldwide [3]. MicroRNAs (miRNAs) are noncoding RNAs 18–25 nucleotides in length [4]. The function of miRNA as posttranscriptional regulation of gene expression by either degradation of the targeting protein coding RNAs or inhibition of their translation into protein, the exosomal miRNAs in body fluids maybe useful diagnostic biomarker for the detection of the cancer [1,5].
IFN is a family of cytokines critical not only for viral interference, but this cytokine can be inhibits cell proliferation and modulates differentiation, apoptosis and migration [6]. An increasing quantity of researches concentration on microRNAs (miRNAs) which play multiple roles in variety of biological processes, including cancer [7]. Most of CCA are unresectable when discovered since it has progressed into advanced stages. So the improvement in the diagnostic method of CCA is urgent, especially in biomarkers [8].
This literature review provides an overview of the most important scientific literature on CCA and exosomal miRNAs. Some scientific systematic reviews have been published in CCA such as cholecystectomy and the risk of cholangiocarcinoma, Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma, preoperative biliary drainage in hilar cholangiocarcinoma, percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma and identification of microRNAs as biomarkers for cholangiocarcinoma detection. However there is no qualitative scientific systematic review of circulating exosomal miRNAs as biomarkers in cholangiocarcinoma. Therefore, reviewers concentrated on the scientific systematic review on circulating exosomal miRNAs serves as candidate biomarker in cholangiocarcinoma.
Materials and Methods
Searching strategy
Reviewers are using the Cochrane guidelines to do a systematic review. The searching strategies used to conduct a systematic computerized search of the PubMed, Science Direct and Google Scholar databases [9]. However, in this review, reviewers accessed publication papers through Medline, EMBASE, and CINAHL to search the articles. Searching term that used as follows (i) “ cholangiocarcinoma” or “intra-hepatic” or “extra-hepatic”; “liver cancer” (ii) “ Circulating microRNA” or “ Exosomal microRNA” or “ miRNA” or “micro-RNA” or “short RNA” or “ small RNA” or “ non-coding RNA” (iii) “ clinical trials” or “ treatment” or “ diagnosis” or “ prognosis” or “recurrence”. A detailed search strategy and search algorithms are shown in the searching results and summary tables.
Inclusion criteria
Research, review and systematic review articles were from trustworthy journals. These scientific articles involved studies reporting data from published papers including cholangiocarcinoma, intra-hepatic CCA, extra-hepatic CCA, liver cancer, pathobiology of biliary epithelial and human bile contain miRNAs extracellular vesicles. The inclusion criteria include: (1) studies must describe in human cholangiocarcinoma; (2) data on the intra-hepatic and extra-hepatic CCA; (3) data related exosomal microRNA and extracellular vesicle of cholangiocarcinoma; (4) data related liver cancer.
Exclusion criteria
In the exclusion, duplicate papers were removed, mismatch papers excluded based on the titles and abstract, and remain papers assessed for eligibility and included in the studies. Thus, the reviews exclude incredible publication papers on other types of cancer.
Review process
Research articles were identified from searches of the electronic databases was imported into ENDNOTE software version X8 (Thomson Reuters, USA). Before the data were extracted, selected articles were read the title and abstract to fulfill the inclusion criteria.
Data extraction and quality assessment
The inclusion and exclusion criteria were used to find the articles based on titles and abstracts. The selected articles were extracted and collected independently. The extraction data were included of the publication data (Authors, year of study, country of study, sample number, tumor stages, microRNA identified, follow-up months, detected samples, assay methods, normalizer RNA).
Results
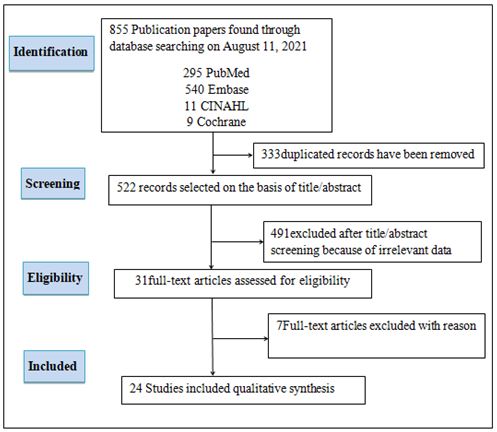
The searching strategy was using Medline, EMBASE, CINAHL, and Cochrane. A total of 855 articles were searched, 333 duplicates removed and remain 522 articles, 491 excluded after screened the titles and abstracts, 4 full text articles were excluded with reason (the article study about long-non coding RNA). 27 articles were included in the studies (Figure 1).
FIG.1. The searching strategy in the systematic review, searching papers using MEDLINE, EMBASE, CINAHL and Cochrane, duplicates papers were removed. The papers screened based on the titles and abstracts and irrelevant excluded. Relevant papers assessed for eligibility (Some irrelevant articles excluded with reason) included articles in the studies.
A total of 30 articles, 18 were focused on CCA, 8 studies were related CCI, 1 study was concentrated on both ICC and ECC. Detected samples and tumor staging were assessed by molecular Real Time-qPCR techniques. List of miRNAs identified from the selected articles as shown in Figure 2 and summary description as indicated in Table 1.
|
No |
Identification |
Year of publication |
Country of study |
Clinical samples |
Biomarker category |
Target genes |
Tumor type |
Assay method |
MicroRNAs identified |
Regulation |
Control |
|
1 |
Bernuzzi F, et al. |
2016 |
UK |
Serum |
Diagnostic |
CEA |
PSC and CCA |
qRT-PCR |
miR-222, miR-483-5p, miR-194 |
Up |
Healthy |
|
2 |
Chen L, et al. |
2009 |
China |
Serum |
Diagnostic |
pCEA |
ICC |
qRT-PCR |
miR-21, miR-25, miR-223 and miR-222 and miR-198 |
Up and Down |
Healthy |
|
3 |
Chen qiabao, et al. |
2015 |
China |
Serum |
Prognostic |
CEA |
CCA |
qRT-PCR |
miR-106a |
Down |
Healthy |
|
4 |
Chusorn P, et al. |
2013 |
Thailand |
Cell line |
Diagnostic |
PDCD4 |
ICC |
qRT-PCR |
miR-21 |
Up |
GAPDH |
|
5 |
Gallego C, et al. |
2016 |
USA |
Plasma |
Diagnostic |
PDCD4 |
ICC |
qRT-PCR |
miR-21, miR-34, miR-200b, miR-221 |
Up |
Healthy |
|
6 |
Fan, et al. |
2017 |
China |
Tissue |
Diagnostic |
iRBE |
ICC |
qRT-PCR |
miR-26b-5p |
Down |
Healthy |
|
7 |
Qian Huan, et al. |
2013 |
China |
Tissue |
Diagnostic |
RBE |
CCA |
qRT-PCR |
miR-21 |
Up |
U6 |
|
8 |
Kawahigashi, et al. |
2009 |
Jepang |
Cell line |
Diagnostic |
HuCCT1, MEC, |
ICC |
qRT-PCR |
miR-22, miR-127, miR-125a |
Down |
U6 |
|
|
TFK-1, IHGGK |
||||||||||
|
9 |
Kim, et al. |
2016 |
South Korea |
SNU-245 |
Diagnostic |
SRGAP2, Rac1. |
ECC |
qRT-PCR |
miR-145-5p |
Up |
Normal |
|
10 |
Kwon, et al. |
2017 |
South Korea |
Serum |
Diagnostic |
Notch1, Notch2,Jagged1 |
CCA |
qRT-PCR |
miR-34a |
Down |
GAPDH |
|
11 |
Li Haoet, et al. |
2017 |
China |
Tissue |
Diagnostic |
TET1-p53 |
ICC |
qRT-PCR |
miR-191 |
Up |
GAPDH |
|
12 |
Li Jingjing, et al. |
2015 |
China |
Tissue |
Prognostic ,Diagnostic |
miR-203 |
CCA |
qRT-PCR |
miR-203 |
Up |
U6 |
|
13 |
Lin zheng, et al. |
2016 |
China |
NP |
NP |
NP |
ICC |
NP |
NP |
NP |
NP |
|
14 |
Liang, et al. |
2016 |
China |
Tissue |
Prognostic |
NP |
CCA |
Pubmed and Embasse |
NP |
NP |
NP |
|
15 |
Chen yaqing, et al. |
2017 |
China |
Tissue |
Prognostic |
TIAM1 |
ICC |
Microarray data |
miR-21 |
NP |
NP |
|
16 |
Liu Ning, et al. |
2015 |
China |
Tissue |
Diagnostic |
RBE |
CCA |
qRT-PCR |
miR-122 |
Down |
Normal |
|
17 |
Lin zheng, et al. |
2016 |
China |
Cell line |
Diagnostic |
RBE |
CCA |
qRT-PCR |
miR-21 |
Down |
GAPDH |
|
18 |
Piontek and Selaru |
2015 |
USA |
NP |
NP |
CCA cells |
CCA |
NP |
NP |
NP |
NP |
|
19 |
Razumilava, et al. |
2012 |
USA |
Cell line |
Diagnostic |
MCM7 |
CCA |
qRT-PCR |
miR-25, miR-106b |
Up |
Normal |
|
20 |
Selaru, et al. |
2009 |
USA |
Tissue |
Diagnostic |
PDCD4 |
CCA |
qRT-PCR |
miR-21 |
Up |
U6 |
|
21 |
Stutes, et al. |
2009 |
USA |
NP |
NP |
CCA cells |
CCA |
NP |
NP |
NP |
NP |
|
22 |
Wang li-juang, et al. |
2015 |
China |
Serum, cell line |
Diagnostic |
RBE, HUCCT1 |
ICC |
qRT-PCR |
miR-21 |
Up |
U6 |
|
23 |
Wang shouli, et al. |
2014 |
China |
Tissue |
Diagnostic |
CEA |
ICC |
qRT-PCR |
miR-150, miR-638 |
Down |
Negative |
|
24
|
Zhang, et al. |
2015 |
China |
Tissue |
Diagnostic |
CEA |
ICC |
qRT-PCR |
miR-612, miR-105-5p |
Down, Up |
|
|
Remark: CEA=Carcinoembryonic antigen, PSC=primary sclerosing cholangitis, CTR=Control, PDCD=Programmed Cell Death, GAPDH=Glyceraldehyde-3-phosphate dehydrogenase, RBE =………. iRBE=FNH=focal nodular hyperplasia |
|||||||||||
Table 1. Summary table of selected papers include in the systematic review.
Discussions
The miRNAs, a class of non-coding regulatory RNAs, have been detected in a variety of organism ranging from ancient unicellular eukaryotes to mammals. MicroRNAs (miRNAs) are non-coding RNAs of 20-22 nucleotides and regulate the translational inhibition of target mRNAs by base-pairing with their 3’-untranslated region (3’-UTR) [10].They have been associated with numerous molecular mechanisms involving developmental, physiological and pathological changes of cells and tissue [11]. Therefore, tissue, serum and plasma samples are required to investigate the existence of miRNAs. The miRNAs itself have been used as biomarkers to diagnose the infectious diseases, parasite, bacteria, including cancers. However, detection of miRNAs as biomarker in infectious diseases and cancers to evaluate the effectiveness of the drugs and diseases progression are still limited.
How microorganisms cause cancers
Helicobacter pylori are commonly transmitted person-to-person by saliva. The bacteria can also be spread by fecal contamination of food or water. Cheng-yeng kao et al. reported that after entering the host stomach, H. pylori utilize its urease activity to neutralize the hostile acidic condition at the beginning of infection [12]. Schwabe RF, et al. stated namely H.pylori infection substantially contributes to global cancer mortality [13]. Meanwhile, Ringehan M, et al. described that Hepatitis B virus (HBV) is one of the choric infection that leading cause for hepatocellular carcinoma (HCC) worldwide [14]. Furthermore, Sithithaworn P, et al. defined explicitly that liver fluke infection causes pathological changes mainly to the bile ducts where the worm can be found, as well as to the liver and gall bladder in both human and animal [15]. The early pathological changes consisted of an acute inflammatory reaction involving the large intra hepatic bile ducts and portal connective tissue. Therefore, the infection of the bacteria, viruses and parasites may produce specific miRNAs during the infection process that leading to cancers.
MicroRNA in cancer
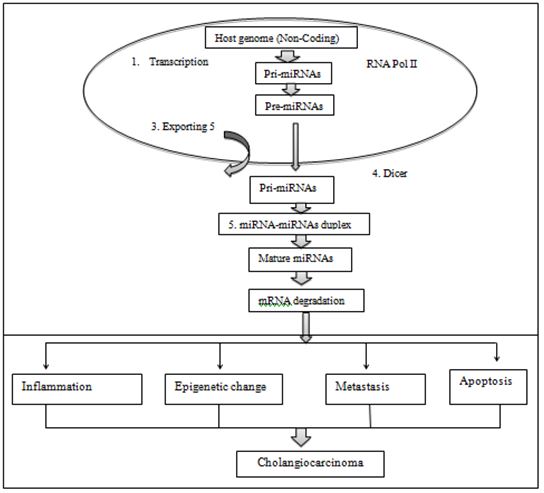
In human, microorganism (Bacteria, virus, parasites) invasion associated gene expression and immune system which is related with diseases severity and assessed drug effectiveness. Li Pang et al. declared that or the expression, miRNAs are first transcribed by RNA polymerase II and processed to form hairpin-like intermediates called pre-miRNAs [16]. Bohnsack MT et al. reported Exportin-5 then transports these pre-miRNAs out of the nucleus and into the cytoplasm for further processing, converting the pre-miRNA into conserved [17]. Bartel DP, et al. defined It is these mature miRNAs of 18-25 nucleotides in length that can control cellular function through recognition of specific targets by complementary base pairing, forming either RNA-induced silencing complexes (RISC), inducing mRNA degradation or inhibiting mRNA translation endogenous, small noncoding mature RNAsas showed in the Figure 2 [18].
FIG.2. Biogenesis of miRNA. (1) DNA transcription and non-coding genome region. (2) Primary-miRNA (Pri-miRNA) transcribed by RNA polymerase II. (3) Dorsha enzyme cut precursor miRNA (Pre-miRNA) and pre-miRNA transferred out of nucleus into cytoplasm with exporting 5. (4) Dicer cut pre-miRNA convert become duplex intermediate. (5) Duplex transform developed into maturemiRNA. (6) MiRNAs degradation. (7) Infected cells and immune system produce cytokines in early infection, severe and cerebral malaria. (8) MiRNAs suppress the expression of the target genes via mRNA cleavage or translation repression. The functions of miRNAs are involved in inflammation, epigenetic change, metastasis, and apoptosis of the cell to promote to CCA cells.
Gabay M, et al. defined MYC is a transcription factor that modulates the gene expression of thousands of genes that regulate many programs which are hallmarks of cancer including: metabolism, proliferation, self-renewal, and survival [19]. Dhanasekaran R, et al. defined MYC’s ability to maintain proliferation, survival and self renewal were regulated via its induction of miR17 ~ 92 clusters [20]. Rangel G. et al. stated candidate miRNAs could be investigated using 3 different algorithms that are the most widely used in the updated version as follows: miRanda, RegRNA and Target Scan [21]. Kanaoka R, et al. showed recent studies have indicated that miRNAs can be detected in biological fluids such as plasma and serum and hold promise as noninvasive biomarkers for cancer patients [22]. Jiao D, et al. describedthe differential miRNA expression patterns were validated with the TaqMan qRT-PCR assay [23].
Candidate miRNAs in cholangiocarcinoma
The miRNAs are a group of small non-coding RNAs, which can bind to mRNAs of target gene leading to their degradation or translational inhibition. Chiang NJ, et al. defined that the roles of miRNA in the biology of CCA, such as proliferation, invasion, migration, differentiation and apoptosis [24]. Rizvi S, et al. reported that CCAs are epithelial tumors with markers of cholangiocyte differentiation; anatomically CCAs are classified into intrahepatic (iCCA), perihilar (pCCA) and distal (dCCA) subtypes [25]. Dutta S, et al. indicated that exosomes are small (40–100 nm in diameter) membrane bound vesicles that are initially formed within the endosomal compartment and secreted upon fusion of the limiting membrane eofmulti vesicularbodies (MVBs) with plasma membrane [26]. Kongpetch S, et al. identified that miRNA in CCA cell lines (HUCCT1 and MEC) revealed biliary epithelial cell-specific miRNAs, i.e., miR22, miR125a, miR127, miR199a, miR199a, miR214, miR376aand miR424, which are downregulated in these lines [27]. Selaru FM, et al. detected in a separate study, miR21 was found to be up-regulated in ICC compared to normal epithelial bile duct tissue. Inhibition of miR21 was shown to increase protein expression of PDCD4 and TIMP3 which are the inhibitors of program-cell death and metastasis, respectively [4].
Candidates’ genes that associated with miRNAs such as FOXA1 associated with miR-212 reported by Zhou J, et al. Zbtb7a associated with miR-106a informed by Jiao et al.; miR-21 associated with PDCD4 as reported by Selaru FM, et al. [4]. The mentioned genes have studied and showed that FOXA1 was correlated with miR-212 in the intra hepatic cholangiocarcinoma; Zbtb7a was linked with miR-106a which the studied was focused on miRNA expression and sensitivity of CCA cell. However, in this scientific systematic review, reviewers will study the candidates’ miRNAs in both intrahepatic CCA and extra-hepatic CCA in the future perspective.
A 24 selected articles were included in the studies, microorganism infection, cell proliferation, migration and differentiation might be produced specific miRNAs which associated with PDCD4 and other genes such as iRBE, RBE, CEA, pCEA, TETI-p53, Rac1were showed that the majority of genes that associated with miRNAs (miR-21, miR-221, miR-26-5p, miR-451, miR-150, miR-191 and miR-122) were detected during the infection, cell proliferation, cell differentiation and promoted tumor invasion and metastasis. These miR-150, miR-191, miR-26, miR-21, miR-451 and miR-122 were associated with ICC samples after detected by molecular PCR. The miR-21 also identified from ECC sample. These miRNAs would be selected as candidates of miRNAs as biomarker of human CCA in the future perspective.
MicroRNA 21
There were 6 studies assessing miR-21 to detect this miRNA expression and its regulates and promoting cell proliferation, cell development and metastasis in CCA [4,28-33]. Gallego C, et al. used 12 human tissue samples to compare with 40 normal plasma specimensin RT-qPCR. The result indicated that there were 4 miRNAs (miR-21, miR-34c, miR-200b, and miR-221) highly up-regulated in the tumor samples [29]. These miRNAs recommended for further analysis in samples. Garajova I, et al. isolated 69 samples from ICC and ECC. Real Time PCR was used to investigate the specimens [30]. The result has shown that miR-21 expression as prognostic biomarker. Therefore, further investigation of ICC and ECC in plasma. Qiu Huang et al. identified miR-21 was significantly higher perineural invasion and lymph node metastasis. The samples were used qRT-PCR for studied. Liu et al. stated namely CCA tumor cell line was isolated and cultured. RNA was reverse transcribed into cDNA using Primer Script RT reagent kit (Takara, China). The result presented miR-21 regulates biological behavior by inducing EMT in human CCA. Selaru FM, et al. argued that 20 primary CCA were compared with 14 normal tissues, using qRT-PCR detect miR-21 was shown highly expressed [4]. Wang L, et al. reported that expression of miR-21 in culture media ICC lines and serum plasma compared with healthy control subjects, using RT-PCR identify miR-21 was shown promoted ICC proliferation and cell growth in vitro by targeting PTPN14 and PTEN [33].
MicroRNA-150
There are 3 studies that assessed miR-150 in CCA to detect the role of the miRNA itself as tumor suppressor gene involved tumor invasion and metastasis, and regulates cell proliferation and cell differentiation [1,34,35]. An Fan F, et al. reported that miR-150-5p continuously decreased in the serum of sclerosing cholangitis and body fluids of CCA. Microarray was used to analyze the serum samples [34]. The results showed that over expression of miR-150 attenuated the growth, invasion and migration capability of CCA cells with target oncogene Ets like gene-1 (ELK1). Wang LJ, et al. described expression miR-150 in tissues and plasma sample from patients compared with normal tissues using quantitative reverse transcription polymerase chain reaction (qRT-PCR) indicated miR-150 patient’s plasma sample was up-regulated with noncancerous plasma [33]. Wu X, et al. stated 28 CCAs and 30 samples from primary sclerosing cholangitis (PSCs) were compared with 50 healthy controls. Serum, bile and tissues samples were used to diagnose in qRT-PCR. The result presented that miR-150 lower expression if compared normal plasma controls [1].
MicroRNA 191 and miR-451
A study (Li et al., 2017) described namely 84 samples from ICC evaluated by RT-PCR indicated that miR-191 up-regulated expressed from ICC tissues compared with adjacent bile duct tissues. Thus, miR-191 also promoted cholangiocarcinoma cell proliferation in human. On one hand, 26 clinical tissue patients were investigated by RT-PCR. The result designated that miR-451 under-expressed compared to the peritumoral tissues.
Conclusion and Prospective
MicroRNAs are small non-coding RNAs that regulate gene expression. They exert their effects on the cells they are synthesized in, and are also released into the extracellular space and transported in body fluids such as blood and urine. Exosomal miRNAs may have important functions in cell-cell communication and have potential as biomarkers to detect and monitor disease. Limited prognosis in CCA as one of the most lethal malignant disease. Thus, low study cover on miRNA in CCA itself. Therefore, reviewers decide 4 candidate miRNAs such as miR-21, miR-150, miR-191 and miR-451 as exosomal circulating miRNAs. Further investigation of the plasma, serum and tissues samples in the laboratory needed performed using real time PCR towards selected candidate miRNAs especially for ICC and ECC samples in the future perspective.
References
- Wu X, Xia M, Chen D, et al.. Profiling of down regulated blood-circulating miR-150-5p as a novel tumor marker for cholangiocarcinoma. Tumor Biol. 2016; 37(11):15019-15029.
- Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Prac Res Clin Gastroenterol. 2015; 29(2):277-293.
- Lv H, Lv G, Han Q, et al. Noncoding RNAs in liver cancer stem cells: The big impact of little things. Cancer letters. 2018; 418:51-63.
- Selaru FM, Olaru AV, Kan T, et al. MicroRNAâ?ÂÂ21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009; 49(5):1595-1601.
- Ogata-Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PloS one. 2014; 9(4).
- Fiorucci G, Chiantore MV, Mangino G, et al. MicroRNAs in virus-induced tumorigenesis and IFN system. Cyt Growth Rev. 2015; 26(2):183-194.
- Huang Q, et al. Circulating microRNAs in colorectal cancer. J Mol Genet Med. 2017; 11274: 1747-1862.
- Zheng, B, Jeong S, Zhu Y, et al. miRNA and lncRNA as biomarkers in cholangiocarcinoma (CCA). Oncotarget. 2017; 8(59):100819.
- Bhagavathula AS, Seid MA, Tegegn HG, et al. Anti-malarial treatment outcomes in Ethiopia: a systematic review and meta-analysis. Malaria J. 2017; 16(1):269.
- Li J, Gao B, Huang Z, et al. Prognostic significance of microRNA-203 in cholangiocarcinoma. Int J Clin Exp Pathol. 2015; 8(8):9512.
- Judice CC, Bourgard C, Kayano ACAV, et al. MicroRNAs in the host-apicomplexan parasites interactions: A review of immunopathological aspects. Frontiers Cellular Inf Microbiol. 2016; 6: 5.
- Chen Y, Liu D, Liu P, et al. Identification of biomarkers of intrahepatic cholangiocarcinoma via integrated analysis of mRNA and miRNA microarray data. Mol Med Reports. 2017; 15(3):1051-1056.
- Schwabe RF, Jobin C. The microbiome and cancer. Nature Rev Cancer. 2013; 13(11): 800-812.
- Ringehan M, McKeating JA, Protzer U, et al. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017; 372(1732):20160274.
- Sithithaworn P, Yongvanit P, Duenngai K, et al. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014; 21:301-308.
- Li P, Ou Q, Braciak TA, et al. MicroRNA-192-5p is a predictive biomarker of survival for Stage IIIB colon cancer patients. Japanese J Clin Oncol. 2018; 48(7):619-624.
- Bohnsack MT, Czaplinski K, Gorlich D, et al. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. Rna. 2004; 10(2):185-191.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136(2): 215-233.
- Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harbor Perspectives in Med. 2014; 4(6):a014241.
- Dhanasekaran R, Gabay-Ryan M, Baylot V, et al. Anti-miR-17 therapy delays tumorigenesis in MYC-driven hepatocellular carcinoma (HCC). Oncotarget. 2018; 9(5):5517.
- Rangel G, Teerawattanapong N, Chamnanchanunt S, et al. Candidate microRNAs as Biomarkers in Malaria Infection: A Systematic Review. Curr Mol Med. 2020; 20(1):36-43.
- Kanaoka R, Iinuma H, Dejima H, et al. Usefulness of plasma exosomal microRNA-451a as a noninvasive biomarker for early prediction of recurrence and prognosis of non-small cell lung cancer. Oncology. 2018; 94(5): 311-323.
- Jiao D, Yan Y, Shui S, et al. miR-106b regulates the 5-fluorouracil resistance by targeting Zbtb7a in cholangiocarcinoma. Oncotarget. 2017; 8(32):52913.
- Chiang NJ, Shan YS, Hung WC, et al. Epigenetic regulation in the carcinogenesis of cholangiocarcinoma. Int J Biochem Cell Biol. 2015; 67:110-114.
- Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma." Journal of hepatology. 2017; 67(3):632-644.
- Dutta S, Reamtong O, Panvongsa W, et al. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015; 1852(9):1989-1999.
- Kongpetch S, Jusakul A, Ong CK, et al. Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways. Best Prac Res Clin Gastroenterol. 2015; 29(2):233-244.
- Zhu L, Huang F, Deng G, et al. MicroRNA-212 targets FOXA1 and suppresses the proliferation and invasion of intrahepatic cholangiocarcinoma cells. Exp Therap Medicine. 2017; 13(5): 2109-2109.
- Correa-Gallego C, Maddalo D, Doussot A, et al. Circulating plasma levels of microRNA-21 and microRNA-221 are potential diagnostic markers for primary intrahepatic cholangiocarcinoma. PloS one. 2016; 11(9).
- Garajova I, Brandi G, Biasco G, et al. MiR-21 expression as prognostic biomarker in extrahepatic but not intrahepatic radically resected cholangiocarcinomas. Annals Oncol. 2015; 26:vi98.
- Huang Q, Liu L, Liu CH, et al. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pacific J Cancer Preven. 2013; 14(2):829-834.
- Liu N, Jiang F, He TL, et al. The roles of MicroRNA-122 over expression in inhibiting proliferation and invasion and stimulating apoptosis of human cholangiocarcinoma cells. Scientific reports. 2015; 5:16566.
- Wang LJ, He CC, Sui X, et al. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget. 2015; 6(8): 5932.
- Fan F, Lu J, Yu W, et al. MicroRNA-26b-5p regulates cell proliferation, invasion and metastasis in human intrahepatic cholangiocarcinoma by targeting S100A7. Oncology Letters. 2018; 15(1):386-392.
- Li H, Zhou ZQ, Yang ZR, et al. MicroRNAâ?ÂÂ191 acts as a tumor promoter by modulating the TET1–p53 pathway in intrahepatic cholangiocarcinoma. Hepatol. 2017; 66(1):136-151.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.