Nanomedicine and Nanoscience Technology: Open Access

Research Article - (2022) Volume 2, Issue 1
Computational Analysis on MicroRNAs that Modulate Significant Host Response Genes as Potential Biomarkers in Cerebral Malaria Infection
2Department of Clinical Microscopy, Mahidol University, Thailand
3Department of Medicine and Public Health, Center for Excellence in Biomedical Science and Engineering, Ubon Ratchathani, Thailand
Received Date: Nov 23, 2021 / Accepted Date: Jan 24, 2022 / Published Date: Jan 31, 2022
Abstract
Introduction: Pathogenesis of severe malaria and cerebral malaria infection is a major problem that affects human body. Candidate genes related to the pathogenesis of malaria disease such as CD36, IFN-γ, TLR4, PRR15 may associate with host microRNA. The contribution of the malaria pathogenesis with host cytokine responses through the cerebral malaria infection may associate with significant miRNAs serving for biomarkers still remain unclear.
Objectives: This study was focused on prediction of host target miRNAs response genes association with pathogenesis of malaria as a potential biomarker for development of severe malaria and cerebral malaria.
Materials and methods: Two different bioinformatics tools including miRanda and Target Scan were used in the prediction of the host genes associated specifically with miRNA as potential biomarkers of malaria.
Results: The prediction result using bioinformatics tools showed that miR-203a-3p.1, miR-146, miR-155-5p, miR-425-5p, miR-217, miR-153-3p, miR-455-3p.2, miR-223, miR-143-3p, miR-146-5p, miR-216a-5p were able to regulate host genes during the development of severe malaria and cerebral malaria.
Conclusion: The prediction of the host microRNA as potential biomarkers from selected gene CD36, TLR4, IFN-γ and PRR15 using bioinformatics tools designated that associated with definite miRNAs such as miR-146 and miR-155. The circulating microRNAs associated with panels of significant host genes should be further investigated.
Keywords
Malaria, Plasmodium falciparum, Circulating miRNAs, Host genes, Prognostic biomarker
Abbreviations
ICAM-1: Intracellular Adhesion Molecule-1; RBCs: Red Blood Cells; EPCR: Endothelial Protein C Receptor; CD36: Cluster of Differentiation 36; IL: Interleukin; TNF-α: Tumor Necrosis Factor alpha; pRBC: parasitized Red Blood Cell; iRBC: infected Red Blood Cells; UTR: Untranslated Region; miRNAs: Micro Ribonucleic Acids; PRR15: Proline Rich 15; TLR4: Toll-like Receptor 4; SNPs: Nucleotide Polymorphisms
Introduction
Malaria harms the infected host as a consequence of the blood stage infection. Illness results from the host responses to this infection and the increased destruction of both infected and uninfected erythrocytes [1]. Erythrocytes infected by mature stages of the parasite adhere to the cellular endothelium through interaction with human receptors such as Intracellular Adhesion Molecule-1 (ICAM-1), Cluster of Differentiation 36 (CD36), Endothelial Protein C Receptor (EPCR), to non-infected erythrocytes forming ‘rosettes’ or to platelets to form agglutinates malaria infection imposes an antibody ‘footprint’ that will last longer than the infection itself [2]. Plasmodium falciparum infection causes paroxysmal fever that is triggered by strong pro-inflammatory responses involving pyrogenic cytokines such as Interleukin (IL)-1β and Tumor Necrosis Factor alpha (TNF-α). Although inflammatory responses, including interferon gamma (IFN-γ), IL-12, IL-1β, IL-2, and TNF- α, play important roles that facilitate parasite clearance, circulating high levels of these cytokines have been associated with malaria immunopathology [3]. The miRNAs and malaria is a dynamic interaction still incomplete understood. Therefore, authors come up with selected genes such as CD36, IFN-γ, Toll-like Receptor 4 (TLR4) and Proline Rich 15 (PRR15) consider as candidate genes that expressed become miRNAs and can be served as potential biomarkers in malaria infection.
Micro Ribonucleic Acids (miRNAs) are a class of small non-coding endogenous RNA molecules that regulate a wide range of biological processes by post-transcriptionally regulating gene expression [4,5]. The regulatory activity of miRNAs is exerted at the post transcriptional level and it is estimated that they act on up to one third of the protein coding genes [6]. The binding of miRNAs to target mRNA is critical for regulating the mRNA level and protein expression [7]. Extracellular miRNAs present in human body fluids are remarkably stable and are protected from endogenous RNase activity. They can be released from cells in membrane-bound vesicles, which prevent degradation from serum RNase. These vesicles include exosomes which are 50–90 nm and other membrane-bound particles such as micro particles which are much larger at 1 μm. It is generally known that cells release exosomes which harbor miRNAs and other bioactive compounds. These exosomes transfer the RNA contents to modulate biological activity in the recipient cell. It is now accepted that exosomes transport information in the form of RNA and this RNA has an effect on cell activity [8]. This review aims to describe the prognosis of circulation miRNAs as potential biomarker of pathologies associated with malaria infection. The forecasting of the selected genes that associated with particular miRNAs using bioinformatics software’s such as Target Scan and miRanda. Furthermore, biogenesis of miRNAs, circulation of miRNAs, single nucleotide polymorphism in miRNA, SNP selection and miRNAs as biomarker in malaria disease described.
Biogenesis of miRNAs
The biogenesis of miRNA started from pri-miRNAs then undergoes processing by two cellular nucleases, Drosha and Dicer, to generate mature miRNAs. In animals, miRNAs mostly bind to complementary sequences in the 3' Untranslated Region (UTR) of their target mRNAs and negatively regulate gene expression either by translational repression or degradation of the mRNA transcript; two processing events lead to mature miRNA formation in animals. In the first, the nascent miRNA transcripts (pri-miRNA) are processed into ~70-nucleotide precursors (pre-miRNA); in the second event that follows, this precursor is cleaved to generate ~21–25 nucleotide mature miRNAs. MiRNAs transcripts are then processed after their synthesis.
Circulating miRNAs
In human, microorganism (bacteria, virus, parasites) invasion associated gene expression and immune system which is related with diseases severity and assessed drug effectiveness [9]. In systemic infections, the presence of a pathogenic agent often induces a significant change in the profile of circulating miRNAs that facilitates their use as biomarkers of disease establishment and progression. In certain cases, such as viral infections, these changes in circulating miRNAs are associated with the targeted cell, as demonstrated in the acute and chronic hepatitis C virus infection. Parasitic flatworms, such as Schistosoma japonicum induce a differential expression of circulating miRNAs in the liver and elsewhere, in the immune response following malaria infection, with many miRNA known to control cell growth and proliferation, stress responses, and metabolism, as well as gene expression and susceptibility to infection [6]. The liver is a known anti-malaria effector organ, as the site of the pre-erythrocytic development of Plasmodium, where kupffer cells and extrathymic lymphocytes are generated following infection to carry out important protective immune functions, such as phagocytosis of parasitized Red Blood Cell (pRBC) [10]. Cerebral malaria caused by cytoadherence of Plasmodium falciparum infected Red Blood Cells (iRBC) to brain endothelium, several endothelial receptors, including CD36 malaria pathogenesis, serves as biomarkers for disease progression and, most importantly be useful for developing therapeutic uses for miRNA or their inhibitors in patients with severe malaria [10,11]. mRNA synthesis in Plasmodium falciparum is α-amanitin-sensitive, indicating the presence of a typical eukaryotic RNA polymerase II. Bioinformatics approaches predicted the homologues of all the 12 subunits of RNA polymerase II, but failed to identify transcription factors associated with this polymerase since a sequence-based approach was used for the search. This could be attributed to the highly AT-rich genome. Later, secondary structure-based approaches were used to predict the general transcription factors 22, and homologues to most but not all transcription factors were discovered.
Single Nucleotide Polymorphism (SNP) in miRNA
The program miRanda takes into account the degree of complementarity and whether the seed sequence resides in an evolutionarily conserved region. Target scan identifies segments with perfect complementarity to the seed region. RNA Hybrid14 calculates Gibbs free energy to determine favorable binding interactions between miRNAs and mRNA transcripts [12]. Single Nucleotide Polymorphisms (SNPs) in miRNA genes (miR-SNPs) are one example of point mutation (albeit one occurring in the past) that could affect miRNA function in one of three possible ways: altering transcription of the primary miRNA transcript; processing of the pri-miRNA and pre-miRNA and; through their effects on modulation of miRNA-mRNA interactions. As a result miR-SNPs have been associated with different types of cancer, including chronic lymphocytic leukemia, gastric, lung and thyroid carcinoma [13].
SNP selection
For the selection of the SNPs, we use PubMed (www.ncbi.nim.nih.gov) data set. For CD36, TLR4, PRR15, the single nucleotide polymorphism database (dbSNP) builds 150 with position of MAF>=0.01. However, IFN-γ unsuccessfully identified by dbSNP due to the global minor allele’s frequency was absent.
MicroRNAs and biomarker in malaria disease
The content of miRNAs in the host is influenced by host-pathogen interactions, as has been shown for bacteria, viruses and apicomplexan parasites, including malaria parasites. Importantly, cyto-adhesion of Plasmodium falciparum to host receptors can trigger intracellular signals in target cells and potentially affect a wide range of cellular responses regulated by miRNAs, such as the expression adhesion molecules, vascular inflammation and parenchymal damage of bone marrow cells. These previous evidences suggest that Plasmodium falciparum, although not able to produce miRNAs, may manipulate host miRNAs pointing to the potential of these small molecules to not only shed light on the molecular mechanisms involved in severe malaria and sickle cell resistance, but also to assess the degree of vital organ dysfunction associated with parasite sequestration, which is believed to constitute a key pathogenic event in Plasmodium falciparum. Thus, miRNAs secreted by host tissues damaged by Plasmodium falciparum sequestration, for instance at the brain, lung, spleen or liver, might have the potential to be developed into a biomarker of malaria specific severe disease [14]. The Toll-like receptors (TLRs) are well studied in malaria and are stimulated by infected Red Blood Cells (RBCs) and/or parasite-derived metabolites, culminating in the release of pro-inflammatory cytokines such as IFN-γ, IL-12, and TNF-α, as well as nitric oxide, which are important for controlling the acute blood-stage infection. However, an excessive or deregulated inflammatory response may result in severe malaria [15].
Polymorphism in miRNA
The binding of miRNA to mRNA is critical for regulating the mRNA level and protein expression. However, this binding can be affected by single-nucleotide polymorphisms that can reside in the miRNA target site, which can either abolish existing binding sites or create illegitimate binding sites. Therefore, polymorphisms in miRNA can have a differing effect on gene and protein expression and represent another type of genetic variability that can influence the risk of certain human diseases [7]. Polymorphisms in TLR4 and TLR9 are described to be associated with malaria susceptibility and with increased parasitemia in Plasmodium falciparum infection or mixed infections [16]. These genes might be expressed during the transcription and post-transcription. Target mRNA degradation processes are facilitated through Ago protein slicer activity. The fact that mRNAs are reduced with an abundance of miRNAs suggests that miRNAs are responsible for mRNA degradation processes. Loss-of-function mutations of the first two identified miRNAs in C. elegans, lin-4 (abnormal cell lineage-4) and let-7 (lethal-7), caused defects in larvae developmental processes. It has been suggested that lin-4 regulates the early developmental stages, whereas let-7 plays an important role in the late developmental processes in C. elegans and possibly some other animals.
Materials and Methods
Overview of this work
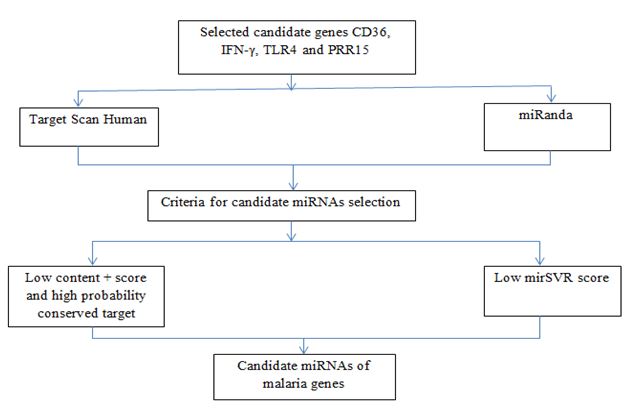
The workflow of this work displayed in Figure 1. Firstly, the selected genes of malaria were found from the related references. Using the selected genes belong to Homo sapiens. Secondly, the genes symbol entered into the target scan software and submitted to get the list of miRNAs that associated with linked genes. The human 3’UTR sequence of the genes were acquired from NCBI database (www.ncbi.nim.nih.gov).
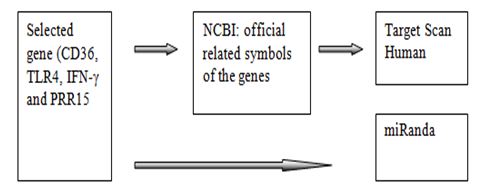
FIG.1. The flow of work processing the selected genes applied into bioinformatics tools.
Prediction of miRNAs
The prediction of miRNAs was investigated using 3 different algorithms that are the most widely used in the updated version as follows: miRanda, RegRNA and Target Scan. However in this work, we use only Target Scan and miRanda to predict the association of the selected genes with specific miRNAs. On the other hand, RegRNA unsuccessfully to be used to predict due to the related genes have overload nucleotides. The workflow of the computational identification of miRNAs involved in malaria was shown in the Figure 2.
FIG.2. The work flow of computational identification of miRNAs.
Target Scan
Target Scan, is used to predict miRNA target sites conserved among orthologous 3UTRs of vertebrate [17-19]. In mammals, prediction are ranked based on the predicted efficacy of targeting as calculated using the content + scores of the site and their probability of conserved targeting. Thus, Target Scan more defined in the whole genome alignments part. However, miRanda preferable the quantity of the Low mirSVR score which represent all miRNAs family. The workflow of the computational identification of miRNAs involved in malaria was shown in Figure 2.
MiRanda
The miRanda software used to detect mirSVR score. Thus, genes were typed into “Target mRNA” column, start prediction by clicking on “go” and “alignment” the prediction result contained with mirSVR score which represent all miRNAs family. The workflow of the computational identification of miRNAs involved in malaria was shown in Figure 2.
MiRNA selection criteria
The selection criteria of miRNA based on the association of the genes and miRNAs indicated during the prediction processes. The selection criteria as follows: (1) exhibited greater than or equal to 2 in 3 prediction tools; (2) high negative free energy that represented more probable hybridization of miRNA-mRNA duplex; (3) high negative mirSVR score showed a high probability of a target inhibition; and (4) high negative content + score and high probability of conserved target revealed good candidate miRNA for target gene inhibition.
Results
We described the prediction of CD36, IFN-γ, TNF-α, TLR4 and PRR15 allied with specific miRNAs using bioinformatics tools. We defined the forecasting of the genes as follows:
Prediction of candidate genes using target scan
The steps of candidate genes (CD36, IFN-γ, TLR4 and PRR15) prediction explained in materials and methods, to identify the association of the genes correlated with particular miRNAs, we used Target Scan software to predict the presence of target miRNA as showed in the Table 1.
|
List of selected candidate genes from Iwalokun et al., 2015 and McGILVRAY et al., 2000 |
|||||||
|
CD36 |
IFN-γ |
TLR4 |
PRR15 |
||||
|
miRNA |
Low content+score |
miRNA |
Low content + score |
miRNA |
Low content + score |
miRNA |
Low content+score |
|
miR-203a-3p.1 |
-0.52 |
miR-24-3p |
-0.56 |
miR-140-5p |
-0.45 |
miR-17-5p |
-0.46 |
|
miR-155-5p |
-0.45 |
miR-29-3p |
-0.38 |
miR-31-5p |
-0.35 |
miR-148 |
-0.44 |
|
miR-425-5p |
-0.38 |
miR-27-3p |
-0.32 |
miR-146-5p |
-0.35 |
miR-155-5p |
-0.23 |
|
miR-217 |
-0.26 |
miR-125-5p |
-0.31 |
miR-7-5p/98-5p |
-0.3 |
miR-140-3p.1 |
-0.22 |
|
miR-153-3p |
-0.25 |
miR-26-5p |
-0.25 |
miR-216b-5p |
-0.27 |
miR-146-5p |
-0.21 |
|
miR-455-3p.2 |
-0.22 |
miR-802 |
-0.22 |
miR-216a-5p |
-0.25 |
miR-101 |
-0.21 |
|
miR-141-3p/200a-3p |
-0.18 |
miR-181-5p |
-0.19 |
miR-34-5p/449-5p |
-0.25 |
||
|
miR-223-3p |
-0.17 |
miR-143-3p |
-0.18 |
Let-217 |
-0.25 |
||
|
miR-124-3p.1 |
-0.15 |
miR-128-3p |
-0.17 |
miR-122-5p |
-0.24 |
||
|
miR-218-5p |
-0.13 |
||||||
|
miR-143-3p |
-0.1 |
||||||
|
miR-221-3p/222-3p |
-0.09 |
||||||
|
miR-146-5p |
-0.09 |
||||||
|
miR-216a-5p |
-0.09 |
||||||
|
miR-204-5p/211-5p |
-0.08 |
||||||
|
miR-194-5p |
-0.07 |
||||||
|
miR-216b-5p |
-0.06 |
||||||
|
miR-143-3p/152-3p |
-0.03 |
||||||
|
miR-203a-3p.1 |
-0.52 |
||||||
|
miR-155-5p |
-0.45 |
||||||
Table 1: The prediction result of candidate genes (CD36, IFN-γ, TLR4 and PRR15) that associated with particular miRNAs using target scan
Prediction of candidate genes using miRanda
Identify the association of candidate genes (CD36, IFN-γ, TLR4 and PRR15) and target miRNAs, we used miRanda software to predict the existence of specific particular miRNA as displayed in the Table 2 and 3.
|
List of selected candidate genes from Iwalokun et al., 2015 and McGILVRAY et al., 2000 |
|||||||
|
CD36 |
IFN-γ |
TLR4 |
PRR15 |
||||
|
miRNA |
Low mirSVR score |
miRNA |
Low mirSVR score |
miRNA |
Low mirSVR score |
miRNA |
Low mirSVR score |
|
miR-329 |
-0.3391 |
miR-761 |
-0.7476 |
miR-145 |
-0.1234 |
miR-24 |
-0.1413 |
|
miR-362-5p |
-0.3391 |
miR-214 |
-0.7476 |
miR-495 |
-0.1234 |
miR-506 |
-0.1413 |
|
miR-137 |
-0.3391 |
miR-223 |
-0.7476 |
miR-329 |
-0.1234 |
miR-146b-5p |
-0.1413 |
|
miR-363 |
-0.3391 |
miR-433 |
-0.7476 |
miR-146b-5p |
-0.1234 |
miR-146a |
-0.1413 |
|
miR-36 |
-0.3391 |
miR-299 |
-0.7476 |
miR-448 |
-0.1234 |
miR-155-5p |
-0.1413 |
|
miR-32 |
-0.3391 |
miR-143 |
-0.7476 |
miR-216b |
-0.1234 |
miR-324 |
-0.1413 |
|
miR-25 |
-0.3391 |
miR-30ca |
-0.7476 |
miR-590-5p |
-0.1234 |
miR-225 |
-0.1413 |
|
miR-92b |
-0.3391 |
miR-30b |
-0.7476 |
miR-23b |
-0.1234 |
||
|
miR-92a |
-0.3391 |
miR-30a |
-0.7476 |
miR-23a |
-0.1234 |
||
|
miR-153 |
-0.3391 |
miR-384-5p |
-0.7476 |
miR-181c |
-0.1234 |
||
|
miR-448 |
-0.3391 |
miR-30d |
-0.7476 |
miR-181a |
-0.1234 |
||
|
miR-106b |
-0.3391 |
miR-30e |
-0.7476 |
||||
|
miR-106a |
-0.3391 |
miR-381 |
-0.7476 |
||||
|
miR-20a |
-0.3391 |
miR-23b |
-0.7476 |
||||
|
miR-17 |
-0.3391 |
miR-23a |
-0.7476 |
||||
|
miR-93 |
-0.7476 |
||||||
|
miR-155 |
-0.7476 |
||||||
|
miR-136 |
-0.7476 |
||||||
Table 2: The prediction result of candidate genes (CD36, IFN-γ, TLR4 and PRR15) that associated with particular miRNAs using miRanda.
|
No.
|
Candidate genes
|
Genes prediction result that associated with specific miRNAs |
|
|
Target Scan |
miRanda |
||
|
1 |
CD36 |
Yes (miR-146 and 155) |
No |
|
2 |
IFN-γ |
No |
Yes (miR-155) |
|
3 |
TLR4 |
Yes (miR-146) |
Yes (miR-146) |
|
4 |
PRR15 |
Yes (miR-146 and 155) |
Yes (miR-146 and 155) |
Table 3: Prediction summary.
MicroRNA candidate production
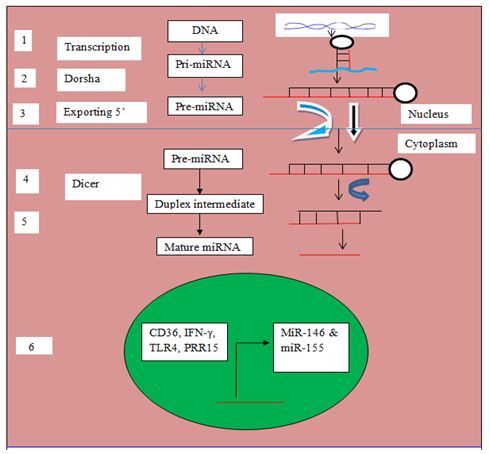
Malaria is an inflammatory disease, which is initiated through recognition of parasite toxin such as glycophosphatidyl inositol (GPI) by innate immune cells such as monocytes, dendritic and macrophages for activation and elicitation of intracellular signal transduction pathway for the production of pro-inflammatory cytokines such tumor necrosis factor-alpha (TNF-α), interleukin-12 (IL-12) and interferon gamma (IFN-γ); Toll-like receptor 4, a major pathogen recognition receptor (PRR) expressed on membrane surface of innate immune cells is genetically encoded by the TLR4 gene. The role in cerebral malaria is unclear because little CD36 is expressed on cerebral microvasculature endothelial cells. Other adhesion receptors such as ICAM-1 are expressed in cerebral endothelial cells and may be up-regulated by inflammatory cytokines such as tumor necrosis factor (TNF)-α. Elevated levels of pro-inflammatory cytokines such as TNF- α. have been associated with severe malaria and a poor prognosis. Therefore, the selected genes (CD36, IFN-γ, TLR4 and PRR15) become candidate miR-146 and miR-155 as biomarker in malaria degradation pathway started from gene transcription in nucleus from pri-miRNA to pre-miRNA after cut by Dorsha and exported into cytoplasm from pre-miRNA to duplex and mature miRNA after cut by dicer as in the Figure 3.
FIG.3. Biogenesis of miRNA. (1) DNA transcription and non coding genome region. (2) Primary –miRNA (pri-miRNA) transcribed by RNA polymerase II. (3) Dorsha enzyme cut precursor miRNA (Pre-miRNA) and pre-miRNA transferred out of nucleus into cytoplasm with exporting 5. (4) Dicer cut pre-miRNA convert become duplex intermediate. (5) Duplex transform developed into mature miRNA (6) candidate genes translated to be miR-155 during transcription processes.
Conclusion and Discussion
Malaria infection stimulates host immune response. Hence, the genes productions were able to use as candidate genes to forecasted using bioinformatics tools. The CD36 was successfully associated with particular miRNAs during the prediction using Target Scan, but unsuccessfully predicted using miRanda. Furthermore, IFN-γ can be predicted using miRanda, ineffectively for Target Scan. Moreover, TLR4 successfully forecasted using both bioinformatics software’s with miR-146 only. Additionally, PRR15 was only the candidate gene can be predicted in both bioinformatics tools with two target miRNAs such as miR-146 and miR-155. Therefore, the potential genes (CD36, PRR15, IFN-γ and TLR4) were associated with particular candidate miRNA (miR-146 and miR-155) become biomarkers in malaria infection.
In summary, this study predicted the host microRNA that related to severe and cerebral malaria infection as potential biomarker from selected gene CD36, TLR4, IFN-γ and PRR15 using bioinformatics tools with specific miRNAs. Interestingly, miR-146 and miR-155 could be a further circulating microRNA contributing to the malaria pathogenesis and cytokine production during the immune responses associated with particular miRNAs served as biomarkers in cerebral malaria infection.
Future plans
To validated clinical samples which are associated with severe and cerebral malaria either its DNA samples or fresh samples to realize the existence circulating microRNAs association with the selected genes such as CD36, IFN-γ, PRR15 and TLR4 on candidate miR-146 and miR-155 should be further studied.
Acknowledgement
The authors thank the College of Medicine and Public Health, Ubon Ratchathani University, Center for Excellence in Biomedical Sciences and Engineering Ubon Ratchathani University Thailand.
References
- White NJ. Malaria parasite clearance. Malar J. 2017; 16(1):88.
- Folegatti PM, Siqueira AM, Monteiro WM, et al. A systematic review on malaria sero-epidemiology studies in the Brazilian Amazon: insights into immunological markers for exposure and protection. 2017; 16(1):107.
- Ademolue TW, Aniweh Y, Kusi KA, et al. Patterns of inflammatory responses and parasite tolerance vary with malaria transmission intensity. 2017; 16(1):145.
- Rangel, G., et al. Candidate microRNAs as Biomarkers in Malaria Infection: A Systematic Review. Current Molecular Medicine 2019; 20(1): 36-43.
- Correia CN, Nalpas NC, McLoughlin KE, et al. Circulating microRNAs as potential biomarkers of infectious disease. Front Immunol. 2017; 8:118.
- De Lacorte Singulani J, De Fátima Da Silva J, Gullo FP, et al. Preliminary evaluation of circulating microRNAs as potential biomarkers in paracoccidioidomycosis. Biomedical Reports. 2017; 6(3):353-357.
- George GP, Mittal RD. MicroRNAs: Potential biomarkers in cancer. Indian J Clin Biochem. 2010; 25(1):4-14.
- Khoury S, Tran N. Circulating microRNAs: potential biomarkers for common malignancies. Biomark Med. 2015; 9(2):131-151.
- Rangel G. Circulating exosomal microRNAs as prognostic biomarkers in Cholangicarcinoma: A systematic review. Nano med Nano Sci Tech. 2021; 1: 1-7
- Cohen A, Combes V, Grau GE. MicroRNAs and malaria-A dynamic interaction still incompletely understood. J Neuroinfect Dis. 2015; 6(1).
- Ohashi J, Naka I, Hananantachai H, et al. Association of PECAM1/CD31 polymorphisms with cerebral malaria. Int J Mol Epidemiol Genet. 2016; 7(2):87-94.
- Preskill C, Weidhaas JB. SNPs in microRNA binding sites as prognostic and predictive cancer biomarkers. Critical reviews in oncogenesis. 2013; 18(4):327-40.
- Chacon-Cortes D, Smith RA, Haupt LM, et al. Genetic association analysis of miRNA SNPs implicates MIR145 in breast cancer susceptibility. BMC Med Genet. 2015; 16:107.
- Rubio M, Bassat Q, Estivill X, et al. Tying malaria and microRNAs: from the biology to future diagnostic perspectives. Malaria J. 2016;15:167.
- Dhangadamajhi G, Kar A, Rout R, et al. A meta-analysis of TLR4 and TLR9 SNPs implicated in severe malaria. Rev Soc Bras Med Trop. 2017; 50(2):153-60.
- Costa AG, Ramasawmy R, Malheiro A, et al. Implications of SNPs on toll-like receptor genes in malaria: What do we know? Rev Soc Bras Med Trop. 2017; 50(2):151-152.
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005; 120(1):15-20.
- Molineaux L. The epidemiology of human malaria as an explanation of its distribution, including some implications for its control. Malaria. 1990: 913-98.
- Rawat M, Bhosale MA, Karmodiya K. Plasmodium falciparum epigenome: A distinct dynamic epigenetic regulation of gene expression. Genom Data. 2016; 7:79-81.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.