Reviews in Pharmacy and Pharmaceutical Sciences

Research Article - (2021) Volume 1, Issue 1
Computational Approach for Designing, Swiss ADME, Molecular Docking and Biological Implication of Nickel Based Transition Metal Complex
Received Date: Sep 26, 2021 / Accepted Date: Oct 22, 2021 / Published Date: Nov 01, 2021
Abstract
In this article was showing a computational approach to statistical learning gives a novel introduction to predictive modeling by focusing on the algorithmic and numeric motivations behind popular statistical methods. In this approach using Swiss ADME web tools for evaluate biophysical parameter like lipophilicity, drug likeness, water solubility and medicinal chemistry of nickel base metal complex data was showing through tabular form. Furthermore, molecular docking study of all compounds was performed against enzymes and DNA DISCOVERY STUDIO 2021 after confirmation of DNA-interaction through docking studies, nuclease activity was performed using agarose gel electrophoresis method and all compounds have been found to cleave DNA. These results concluded that nickel complexes of sulfonamide may be good induction in the future for medical purposes.
Keywords
Computational approach, Swiss ADME, Biophysical parameter, Docking studies
Introduction
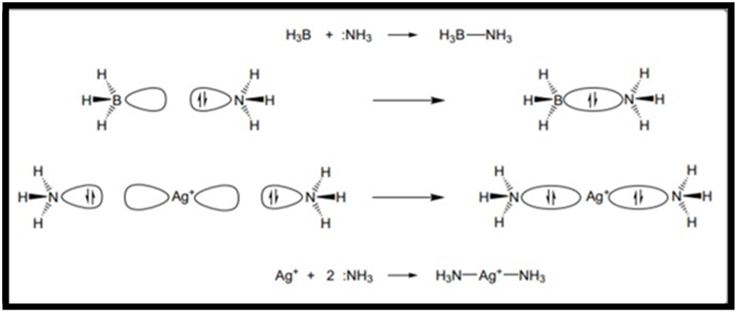
Molecular structure, in its simplest sense, is interpreted in terms of covalent bonds formed through shared pairs of electrons. First introduced by G.N. Lewis almost a century ago, the concept of a covalent bond formed when two atoms share an electron pair remains as a firm basis of chemistry, giving us a basic understanding of single, double and triple bonds, as well as of a lone pair of electrons on an atom. Evolving from these simple concepts came valence bond theory, an early quantum mechanical theory which expressed the concepts of Lewis in terms of wave functions [1-8] . However, coordination chemistry is marked by a need to employ the additional concept of coordinate bond formation, where the bond pair of electrons originates on one of the two partner atoms alone. In most coordination compounds it is possible to identify a central or core atom or ion that is bonded not simply to one other atom, ion or group through a coordinate bond, but to several of these entities at once. The central atom is an acceptor, with the surrounding species each bringing (at least) one lone pair of electrons to donate to an empty orbital on the central atom [9]. The central atom is a metal or metalloid, and the compound that results from bond formation is called a coordination compound, coordination complex or often simply a complex [10-16].
FIG.1. A schematic view of ammonia acting as a donor ligand to a metalloid acceptor and to a metal ion.
Coordination has a range of consequences for the new assembly. It leads to structural change, seen in terms of change in the number of bonds and/or bond angles and distances. This is inevitably tied to a change in the physical properties of the assembly, which differ from those of its separate components. With metal atoms or ions at the centre of a coordination complex, even changing one of a set of ligands will be reflected in readily observable change in physical properties, such as color. With growing sophistication in both synthesis and our understanding of physical methods, properties can often be tuned through varying ligands to produce a particular result, such as a desired reduction potential. It should also be noted that a coordination compound adopts one of a limited number of basic shapes, with the shape determined by the nature of the central atom and its attached ligands. Moreover, the physical properties of the coordination compound depend on and reflect the nature of the central atom, ligand set and molecular shape. Whereas only one central atom occurs in many coordination compounds (a compound we may thus define as a monomer), it should also be noted that there exists a large and growing range of compounds where there are two or more central atoms, either of the same or different types [17-20]. These central atoms are linked together through direct atom-to-atom bonding, or else are linked by ligands that as a result are joined to at least two central atoms at the same time. This latter arrangement, where one or even several ligands are said to bridge between central atoms, is the more common of these two options. The resulting species can usually be thought of as a set of monomer units linked together, leading to what is formally a polymer or, more correctly when only a small number of units are linked, an oligomer. We shall concentrate largely on simple monomeric species herein, but will introduce examples of larger linked compounds where appropriate.
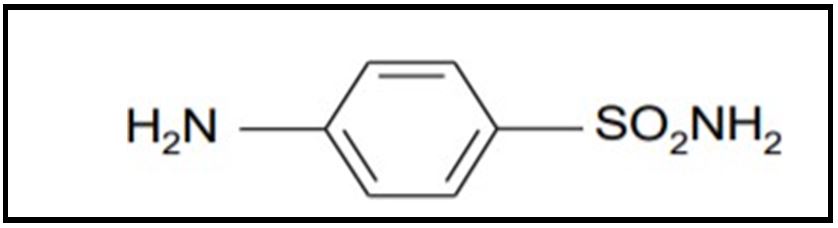
Sulfa moiety
These are synthetic chemotherapeutic agents which contain sulphonamide groups (–SO2 NH2) in their structure. These were the first effective chemotherapeutic agents to be widely used for the cure of bacterial infections in human. Glemo in 1908 synthesized a compound p– aminobenzenosulphonamide commonly known as sulphanilamide for the study of azo dyes. Later on Gerhard Domagk screened a number of these azo dyes for their antibacterial effects and observed that they were active against streptococci. Domagk prepared a red dye 4–sulphonamide–2’,4’–diaminobenzene known as prontosil which has significant curative properties.
FIG.2. Diagram represents the sulphonamide moiety.
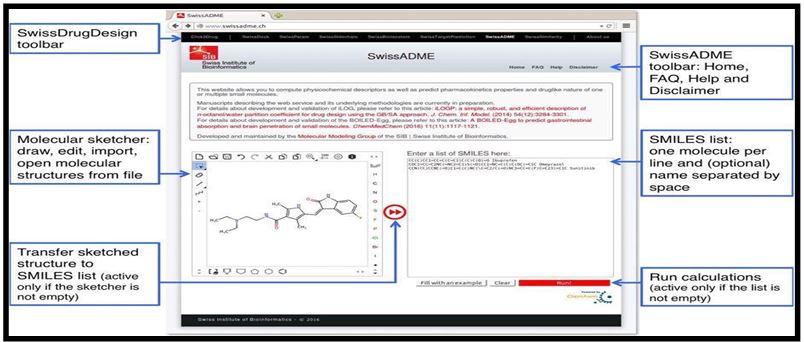
Web tool predictive models Swiss ADME for physicochemical properties
It present the new Swiss ADME web tool that gives free access to a pool of fast yet robust predictive models for physicochemical properties, pharmacokinetics, drug-likeness and medicinal chemistry friendliness, among which in-house proficient methods such as the BOILED-Egg, iLOGP and Bioavailability Radar. Easy efficient input and interpretation are ensured thanks to a user-friendly interface through the login-free website http://www.swissadme.ch. Specialists, but also non expert in chem. informatics or computational chemistry can predict rapidly key parameters for a collection of molecules to support their drug discovery endeavors [21].
Fig.3 Diagram represents the swiss ADME Software in webpage
Density functional theory applied for the study of synthesis and processing parameters
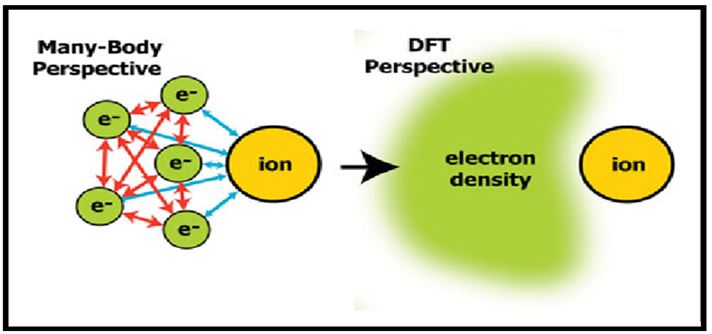
DFT computational methods are applied for the study of systems exhibiting high sensitivity to synthesis and processing parameters [22-23]. In such systems, experimental studies are often encumbered by inconsistent results and non-equilibrium conditions. Examples of contemporary DFT applications include studying the effects of dopants on phase transformation behavior in oxides, magnetic behavior in dilute magnetic semiconductor materials and the study of magnetic and electronic behavior in ferroelectrics and dilute magnetic semiconductors [24,25]. In practice, Kohn Sham theory can be applied in several distinct ways depending on what is being investigated. In solid state calculations, the local density approximations are still commonly used along with plane wave basis sets, as an electron gas approach is more appropriate for electrons delocalized through an infinite solid.
FIG.4. Density functional theory (DFT) abandons the many particle electron.
Molecular docking used to predict the strength of binding affinity
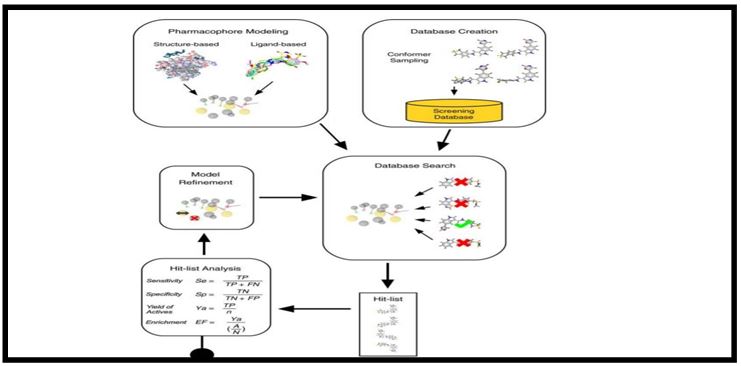
Molecular docking is a method which predicts the preferred orientation of one molecule to a second when bound to each other to form a stable complex Knowledge of the preferred orientation is used to predict the strength of association or binding affinity between two molecules using scoring functions [26-28]. The associations between biologically relevant molecules such as proteins, nucleic acids, carbohydrates, and lipids play central role in signal transduction. Docking is frequently used to predict the binding orientation of drug candidates to their protein targets in order to predict the affinity and activity of the small molecule. Hence docking plays an important role in the rational design of drugs .The aim of molecular docking is to achieve an optimized conformation for both the protein and ligand and relative orientation between protein and ligand so that the free energy of the overall system is minimized. Molecular recognition plays a key role in promoting fundamental bimolecular events such as enzyme- substrate, drug-protein and drug-nucleic acid interactions [29].
FIG.5. 3D Pharmacophore-based virtual screening workflow.
Structural formulation and investigation by Chemdraw software
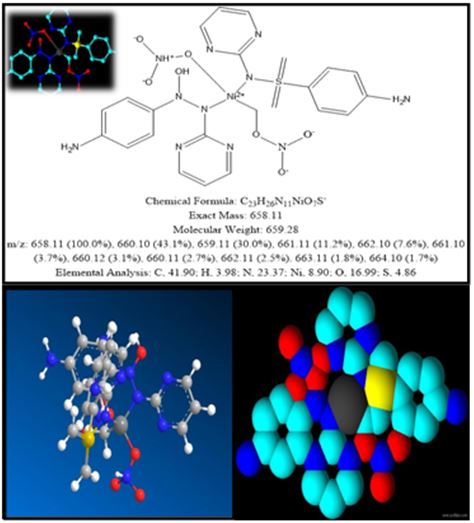
FIG.6. 3D structure of nickel metal complex formulated by Chem3D Software
Biophysical parameter evaluate by Swiss ADME
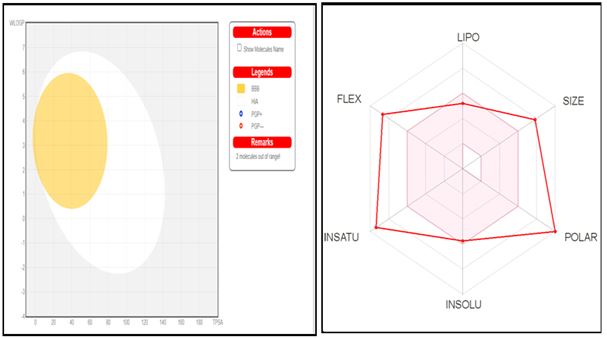
Concomitant predictions for both brain and intestinal permeation are obtained from the same two physicochemical descriptors and straight forwardly translated into molecular design, owing to the speed, accuracy, conceptual simplicity and clear graphical output of the model [30-35]. The BOILED-Egg can be applied in a variety of settings, from the filtering of chemical libraries at the early steps of drug discovery, to the evaluation of drug candidates for development [32].
Fig.7. Biophysical parameters.
| Physicochemical properties | |
|
Formula |
C23H26N11NiO7S |
|
Molecular weight |
659.28 g/mol |
|
Num. heavy atoms |
43 |
|
Num. arom. heavy atoms |
24 |
|
Fraction Csp3 |
0.04 |
|
Num. rotatable bonds |
13 |
|
Num. H-bond acceptors |
12 |
|
Num. H-bond donors |
4 |
|
Molar Refractivity |
160.16 |
|
TPSA |
273.73 Ų |
| Liphophilicity | |
|
Log Po/w (iLOGP) |
0 |
|
Log Po/w (XLOGP3) |
3.58 |
|
Log Po/w (WLOGP) |
1.11 |
|
Log Po/w (MLOGP) |
-2.51 |
|
Log Po/w (SILICOS-IT) |
-8.34 |
|
Consensus Log Po/w |
-1.23 |
| Water solubility | |
|
Log S (ESOL) |
-5.74 |
|
Solubility |
1.21e-03 mg/ml ; 1.83e-06 mol/l |
|
Class |
Moderately soluble |
|
Log S (Ali) |
-9.01 |
|
Solubility |
6.38e-07 mg/ml ; 9.67e-10 mol/l |
|
Class |
Poorly soluble |
|
Log S (SILICOS-IT) |
-2.89 |
|
Solubility |
8.42e-01 mg/ml ; 1.28e-03 mol/l |
|
Class |
Soluble |
| Pharmacokinetics | |
|
GI absorption |
Low |
|
BBB permeant |
No |
|
P-gp substrate |
Yes |
|
CYP1A2 inhibitor |
No |
|
CYP2C19 inhibitor |
No |
|
CYP2C9 inhibitor |
Yes |
|
CYP2D6 inhibitor |
No |
|
CYP3A4 inhibitor |
Yes |
|
Log Kp (skin permeation) |
-7.78 cm/s |
| Druglikeness | |
|
Lipinski |
No; 2 violations: MW>500, NorO>10 |
|
Ghose |
No; 2 violations: MW>480, MR>130 |
|
Veber |
No; 2 violations: Rotors>10, TPSA>140 |
|
Egan |
No; 1 violation: TPSA>131.6 |
|
Muegge |
No; 3 violations: MW>600, TPSA>150, Hacc>10 |
|
Bioavailability Score |
0.11 |
|
Medicinal Chemistry |
|
|
PAINS |
1 alert: anil_no_alk |
|
Brenk |
3 alerts: aniline, oxygen-nitrogen_single_bond, thiocarbonyl_group |
|
Leadlikeness |
No; 3 violations: MW>350, Rotors>7, XLOGP3>3.5 |
|
Synthetic accessibility |
5.62 |
Table 1. Biophysical parameter evaluated by Swiss ADME web tool.
Molecular docking of Nickel based metal complex
An excellent review has been published describing the application of pharmacophore based modeling methods in discovering new leads in the absence of structural data. The success of a docking program depends on two components such as search algorithm and scoring function. Searching conformational space the search space consists of all possible orientations and conformations of the protein paired with ligand [36].
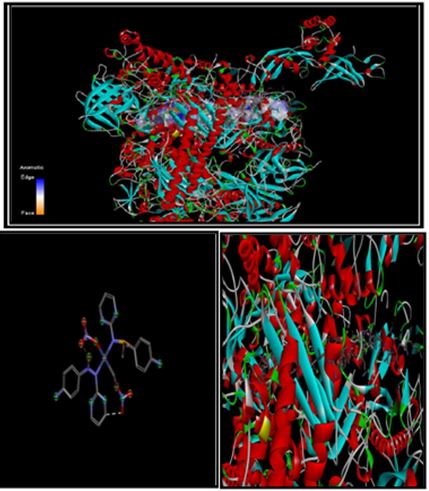
Docking was performed with discovery studio visualize software and receptor was taken online protein data bank (https://www.rcsb.org) 7OL0 Structure of active transcription elongation complex Pol II-DSIF (SPT5-KOW5) main protease pdb. Nickel containing complex of sulphonamides shows least energy of bind site as mentioned in the above figure in the blue spot (seven binding site) with receptor that is known proteas. Nickel (II) complexes of sulfonamides have found importance in biological and pharmaceutical systems [37-45]. This study focuses on Nickel (II) complexes that were synthesized by precipitation method. The spectroscopic characterization was done by different techniques like Swiss ADME, DFT, PDB.
Furthermore, molecular docking study of all compounds was performed against enzymes and DNA DISCOVERY STUDIO 2021 [38-57]. After confirmation of DNA-interaction through docking studies, nuclease activity was performed using agarose gel electrophoresis method and all compounds have been found to cleave DNA. These results concluded that nickel complexes of sulfonamide may be good induction in the future for medical purposes.
Fig.8. Docking between ligand containing metal complex of Nickel with sulphonamides with 7OL0 of the SARS main protein.
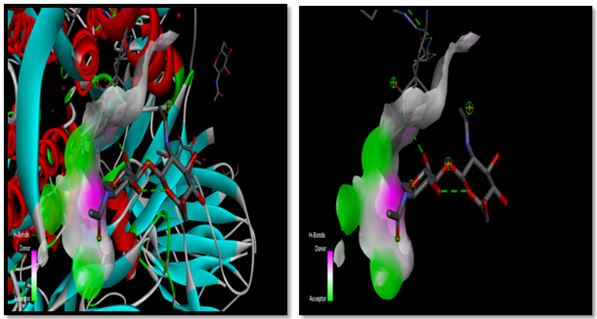
Fig.9. Hydrogen bond interaction of Nickel with sulphonamides with 7OL0 of the SARS main protein.
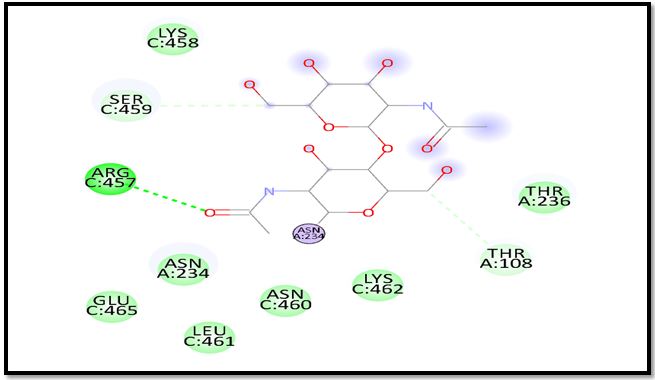
Fig.10. 2D structure of Nickel with sulphonamides with 7OL0 of the SARS main protein.
Conclusion
The overall conclusion in this research article was showing a computational approach to statistical learning gives a novel introduction to predictive modeling by focusing on the algorithmic and numeric motivations behind popular statistical methods. Through this theme, the computational approach motivates and clarifies the relationships between various predictive models. furthermore Concomitant predictions for both brain and intestinal permeation are obtained from the same two physicochemical descriptors and straight forwardly translated into molecular design, owing to the speed, accuracy, conceptual simplicity and clear graphical output of the model. In this approach using Swiss ADME web tools for evaluate biophysical parameter like lipophilicity, drug likeness, water solubility and medicinal chemistry of nickel base metal complex data was showing through tabular form. Furthermore, molecular docking study of all compounds was performed against enzymes and DNA DISCOVERY STUDIO 2021 after confirmation of DNA-interaction through docking studies, nuclease activity was performed using agarose gel electrophoresis method and all compounds have been found to cleave DNA. These results concluded that nickel complexes of sulfonamide may be good induction in the future for medical purposes.
Acknowledgement
Author Mohd.Washid Khan thanks Prof. Kapil deo Mishra, Vice Chancellor, Rani Durgavati University, Jabalpur for providing medium-sized libraries and workplaces. I would also like to inform Dr. Brajesh Singh, Registrar, Rani Durgavati University, Jabalpur Instrumentation Provisioning and Laboratory Facility.
Conflict of Interest
The authors declare no conflict of interest.
References
- Gillespie RJ. Fifty years of the VSEPR model. Coord Chem Rev. 2008; 252: 1315-1327.
- Gillespie RJ. Teaching molecular geometry with the VSEPR model. J Chem Educ. 2004; 81:298-304.
- Knowles PJ, Schütz M, Werner HJ. Ab initio methods for electron correlation in molecules. 2000; 1:69-151.
- Frenking G, Bickelhaupt FM. The EDA perspective of chemical bonding. Theo Chem. 2014; 121-157.
- Frenking G, Krapp A. Unicorns in the world of chemical bonding models. J Comput Chem. 2007; 28:15-24.
- Esterhuysen C, Frenking G. The Nature of the chemical bond revisited. An energy partitioning analysis of diatomic molecules E2 (E=N–Bi, F–I), CO and BF. Theor Chem Acc. 2004; 111:381-389.
- Levine DS, Horn PR, Mao Y, et al. Variational energy decomposition analysis of chemical bonding. 1. spin-pure analysis of single bonds. J Chem Theory Comput. 2016; 12:4812-4820.
- Schoendorff G, Schmidt MW, Klaus Ruedenberg K, et al. Quasi-atomic bond analyses in the sixth period: II. Bond analyses of cerium oxides. J Phys Chem. 2019; 123:5249-5256.
- Duchimaza Heredia JJ, Sadow AD, Gordon MS. A quasi-atomic analysis of three-center two-electron zr−h−si interactions. J Phys Chem A. 2018; 122:9653-9669.
- Duchimaza Heredia JJ, Ruedenberg K, Gordon MS. Quasi-atomic bonding analysis of xe-containing compounds. J Phys Chem A. 2018; 122: 3442-3454.
- West AC, Duchimaza Heredia JJ, Gordon MS, et al. Identification and characterization of molecular bonding structures by Ab initio quasi-atomic orbital analyses. J Phys Chem A. 2017; 121:8884-8898.
- Mayer I. Bond orders and energy components: Extracting chemical information from molecular wave functions. CRC Press; Boca Raton, FL, USA: 2017.
- Dunning TH, Xu LT, Takeshita Y, et al. Insights into the electronic structure of molecules from generalized valence bond theory. J Phys Chem A. 2016; 120:1763-1778.
- Frenking G, Shaik S. The chemical bond: Chemical bonding across the periodic table. wiley-VCH Verlag; Weinheim, Germany: 2014.
- Frenking G, Shaik S. The chemical bond: Fundamental aspects of chemical bonding. Wiley-VCH Verlag; Weinheim, Germany: 2014.
- Weinhold F, Landis CR. Valency and bonding: A natural bond orbital donor-acceptor perspective. Cambridge Univ. Press; Cambridge, UK: 2005.
- Armbruster T, Döbelin N, Peretti A, et al. Am. Mineral. 2004; 89:610-613.
- Bersuker IB. The jahn–teller effect. Cambridge University Press. 2006.
- Bersuker IB. Electronic structure and properties of transition metal compounds: Introduction to the theory. Hoboken, New Jersey, USA: John Wiley and Sons Inc. 2010.
- Bersuker IB. The jahn–teller and pseudo-jahn–teller effects: A unique and only source of spontaneous symmetry breaking in atomic matter. Chem Rev. 2013; 113: 1351-1390
- Bersuker IB. Spontaneous symmetry breaking in matter induced by degeneracies and pseudodegeneracies. Adv Chem Phy. 2016; 160: 159-208.
- Bersuker IB. Vibronic coupling and electron-phonon interactions in molecules and crystals: xxiii international symposium on the jahn-teller effect 27 August to 1 September 2016, Tartu, Estonia. J Phys Conf. 2017.
- Brown ID. A simplified empirical model for approximation of the `bond valence-bond length' correlation for H-O bonds. Chem Rev. 2009; 109: 6858-6919.
- Brown ID. Bond valence thoery. Bond Valences. 2014; 2: 11-58.
- Brown ID. The chemical bond in inorganic chemistry: The bond valence model. Oxford University Press.
- Chen M, Xia Z, Molokeev MS, et al. Chem Mater. 2017; 29: 1430-1438.
- Chi EO, Ok KM, Porter Y, et al. Chem Mater. 2006; 18: 2070-2074.
- Coey JMD. Solid State Sci. 2005; 7: 660-667.
- Fan YH, Jiang XM, Liu BW, et al. Phase transition and second harmonic generation in thiophosphates Ag2Cd (P2S6) and AgCd3(PS4)S2 containing two second-order jahn-teller distorted cations. Inorg Chem. 2017; 56: 114-124.
- Fukuzumi S. fundamental concepts of catalysis in electron transfer. electron transfer in chemistry. 2001; 4; 2-67.
- Gagne OC. Bond-length distributions for ions bonded to oxygen: metalloids and post-transition metals. Acta Cryst. 2018; B74; 49-62.
- Gagné OC. On the crystal chemistry of inorganic nitrides: Crystal-chemical parameters, bonding behavior, and opportunities in the exploration of their compositional space. Chem Rxiv. 2020; 11626974.
- Gagné OC, Hawthorne FC. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015; B71, 562-578.
- Gagné OC, Hawthorne FC. Bond-length distributions for ions bonded to oxygen: Alkali and alkaline-earth metals. Acta Cryst. 2016; B72: 602–625.
- Gagné OC, Hawthorne FC. Empirical lewis acid strengths for 135 cations bonded to oxygen. Acta Cryst. 2017; B73: 956-961.
- Gagné OC, Hawthorne FC. Mean bond-length variations in crystals for ions bonded to oxygen. Acta Cryst. 2017; B73: 1019-1031.
- Gagné OC, Hawthorne FC. Bond-length distributions for ions bonded to oxygen Metalloids and post-transition metals. Acta Cryst. 2018; B74: 63-78.
- Gagné OC, Hawthorne FC. Bond-length distributions for ions bonded to oxygen: results for the non-metals and discussion of lone-pair stereoactivity and the polymerization of PO4. Acta Cryst. 2018; B74: 79-96.
- Gagné OC, Mercier PHJ, Hawthorne FC. Another look at bonds and bonding. Acta Cryst. 2018; B74: 470-482.
- Galy J, Enjalbert R, Rozier P, et al. Ytterbium cobalt gallium oxide, YbCoGaO4, as grown by the floating zone technique. Acta Cryst. 2002; C58: i6–i8.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. J Clin. 2014; 64: 9-29,
- Garcia-Fernandez P, Bersuker IB. Class of molecular and solid state systems with correlated magnetic and dielectric bistabilities induced by the pseudo jahn-teller effect. Phys Rev. 2011; 106: 246406.
- Groom CR, Allen FH. Applications of the cambridge structural database in organic chemistry and crystal chemistry. Chem Int. 2014; 53: 662-671.
- Groom CR, Bruno IJ, Lightfoot MP, et al. The development and use of a crystallographic database. 2016; Acta Cryst. B72: 171-179.
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-482.
- Petri WA Jr. Sulonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s the pharmacological basis of therapeutics. Pharmacology and Therapeutics 2011; 1463-1476.
- Silva D. Synthesis and in vitro evaluation of leishmanicidal and trypanocidal activities of N-quinolin-8-yl arylsulfonamides. Bioorg Med Chem. 2007; 15: 7553-7560.
- Rathisha IG, Javed K, Ahmad S, et al. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg medi chem. 2009; 19(1): 255-258.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. J Clin. 2014; 64: 9-29.
- Newman DJ. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J Med Chem. 2008; 51: 2589-2599,
- Elmsellem H, El Ouadi Y, Mokhtari M, et al. A natural antioxidant and an environmentally friendly inhibitor of mild steel corrosion: A commercial oil of basil (Ocimum Basilicum L.). J Chem Technol Metall. 2019; 54: 742-749.
- Moussaoui ElA, Jawhari FZ, Almehdi AM, et al. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens L. Bioorg Chem. 2019; 1--12.
- Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018; 19: 20-31.
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One. 2017; 14-20.
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012; 75: 311-335.
- Hemshekhar M, Santhosh MS, Kemparaju K. et al. Emerging roles of anacardic acid and its derivatives: A pharmacological overview. Basic Clin Pharmacol Toxicol. 2012; 1-5.
- Hamad F, Mubofu E. Potential biological applications of bio-based anacardic acids and their derivatives. Int J Mol Sci. 2015; 16: 8569-8590.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.