Nanomedicine and Nanoscience Technology: Open Access

Mini Review - (2022) Volume 2, Issue 1
Distribution of Leptons by Van der Waals Radius in Exobiological Nanomolecules
2Faculty of Chemistry, USA
3University Autonomous of Campeche (Faculty of Chemical-Biological Sciences), Calle Av. Agustín Melgar s/n, Buenavista, 24039 Campeche, Mexico
4Department of Marine Science, University of Calcutta, 35 B. C Road, Kolkata, 700019, West Bengal, India
Received Date: May 03, 2022 / Accepted Date: May 15, 2022 / Published Date: May 15, 2022
Abstract
The focus of the work deals with the analysis of the action sites of four exobiological nanomolecules, determined by the distribution of electrical charges around the nanomolecules atoms called: ASi, CSi, GSi and TSi. The Van der Waals radius distribution calculations have been determined via ab initio Hartree-Fock methods, Unrestricted and Restrict (UHF and RHF) in the set of basis used Effective Core Potential (ECP) minimal basis, and CC-pVTZ (Correlation-consistent valence-only basis sets triple-zeta). The study has so far been limited to computational ab initio methods. The results are compatible with the theory of quantum chemistry, but their comprovation experimental verification depend on advanced techniques for their synthesis, obtaining in laboratory for experimental biochemical.
Keywords
Adenine, Cytosine, Guanine, Thymine, Hartree-Fock method, Nanomolecule, CC-pVTZ, Leptons, Van der waals radius, Exobiological
Introduction
On the basis in chemical evolutionary theory, it is implicit that life is being based upon carbon chemistry [1]. The possibility of life based on silicon has been discussed extensively (though casually). Theoretical chemical arguments have been proposed to support this presumption [1].
The most significant result would be to find some type of living matter radically different from that of the Earth. One might cite under this category supposed organisms with a structure and metabolic machinery based on silicon rather than on carbon; or forms with an ammonia based rather than a water-based machinery and metabolism. (One should note in the former case, however, that fully aerobic silicon metabolizers would be required to exhale quartz.) [1].
Exclude the noble gases from consideration because of their inertness; the four most abundant elements of the universe are hydrogen, oxygen, carbon, and nitrogen. In fact, hydrogen is the major constituent of the universe; oxygen, carbon, and nitrogen are each about ten times more plentiful than the next most abundant element, silicon [1].
In comparing the carbon and silicon has: the Si lies in the same column of the periodic table of the elements, and it has been investigated as a possible alternative for building up biological molecules in exobiology [2,3].
The option for simple replacement of carbon by silicon [4,5] is due to the peculiar characteristics between both. Atomic interactions under non-carbon conditions were studied, with only the Hydrogen, Silicon, Nitrogen and Oxygen atoms, in STP (Standard Temperature and Pressure), for the four standard bases of DNA, A, C, G and T, thus obtaining by quantum chemistry four new compounds, named here as: ASi, CSi, GSi and TSi [6-11].
Silicon based chemistry, however, is by far less flexible than carbon chemistry, not able to form double covalent bonds with the same easiness as Carbon does. Other fact is the larger volume occupied by the external electronic orbitals of silicon tend to reduce the superposition of p orbitals [2,3].
Through the chemical abundances of biological elements in the earth crust, terrestrial life has chosen carbon instead of silicon, in spite of the larger abundance of silicon. This fact suggests that carbon is better suited to form biological molecules [2,3]. However, this paper assumes conditions without the presence of Carbon.
Calculations obtained in the ab initio Unrestricted and Restrict Hartree-Fockmethod, (UHF and RHF). The set of basis used Effective core potential (ECP) minimal basis, CC-pVTZ (Correlation-consistent valence-only basis sets triple-zeta) [12-18].Hartree-Fock Methods, Hardware and Software
For calculations the computer used for was a Desktop with SUSE Linux Enterprise Desktop [19], AMD Ryzen 7 1800X processor [20], ASUS [21] Prime A320M-K motherboard, 16GB of RAM, with 500GB SSD [22].
The ab initio [12-18] calculations have been performed to study the equilibrium configuration, and calculation of the Mulliken [12] loads for CC-pVTZ [12] exobiological molecules of the study. The set of programs GaussView 5.0.8 [23], GAMES [17,18], BIOVIA Draw 2017 [24], and CHARMM22 [25,26] were used.
Results
The distribution of leptons in ASi, CSi, GSi and TSi molecules, were obtained through computationally calculated using the ab initio Hartree-Fock (HF) method [6-11].
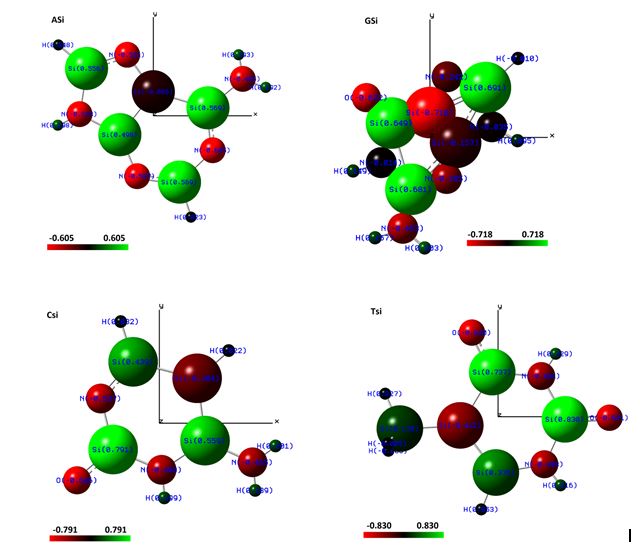
The Figure (1) shows the distribution of Mulliken electrical charges around the atoms of exobiological molecules, using a scale of 0.75 of the Van der Waals radius. With a color gradient going from red, black to green, that is, gradient of negative charges in red, to green, positive charges, respectively.
Fig.1. Representation of the molecular structure of ASi, CSi, GSi and TSi molecules with distribution of Mulliken electrical charges. Images obtained in the software Gaussview, Version 5, 2009 [23].
Molecules Properties
ASi
IUPAC name:
2,3,4,5,6,7,8,9-octahydro-1H-[1-5]diazatrisilolo[4,5-d][1-6]diazatetrasilin-8-amine [6-10,24];
E(RHF): -1719.94065566 a. u. [17,18];
Dipole Moment: 3.2363 Debye [17,18];
Molecular Formula: H13N5Si5.
Nitrogen and Silicon atoms present strong bonding potentials, prone to Hydrogen bonds, due to the shifts of charges of Silicon atoms (cationic), while Nitrogens (anionic). With the exception of Silicon from the central ring bonded to Nitrogen and other Silicon.
CSi
IUPAC name:
2-hydroxy-1,3,2,4,5,6-diazatetrasilinan-4-amine [6-10,24];
E(UHF): -1396.96978499 a. u. [17,18];
DipoleMoment: 10.6516 Debye [17,18];
Molecular Formula: H11N3OSi4.
Oxygen and Nitrogen are anionic and two Silicon atoms (cationic), thus concentrating on these hydrogen bonds.
GSi
IUPAC name:
8-oxo-3,7-dihydro-[1-5]diazatrisilolo [4,5-d] [1,3-,6]diazatetrasilin-6-amine [6-10,24];
E(RHF): -1791.74221629 a. u. [17,18];
Dipole Moment: 9.7172 Debye [17,18];
Molecular Formula: H5N5OSi5.
GSi has the highest dipole moment due to the displacement of electrical charges between the atoms of the molecule. The Oxygen atom is anionic, while three Silicon atoms are cationic, and one anionic. These have strong potential for forming hydrogen bonds.
TSi
IUPAC name:
(2,4-dihydroxy-1,3,2,4,5,6-diazatetrasilinan-5-yl)silane [6-10, 24];
E(RHF): -1706.93799137 a. u. [17,18];
DipoleMoment: 8.7051 Debye [17,18];
Molecular Formula: H12N2O2Si5.
The two oxygen atoms as well as the two nitrogen atoms are anionic as expected, due to the displacement of charges from the predominantly cationic silicons.
Conclusion
Dipole moment of nanomolecules in decreasing order: CSi> GSi>TSi>ASi; E(HF): GSi>CSi>ASi>TSi; and Mulliken Charge Range: TSi>CSi>GSi>ASi.
The study has so far been limited to computational ab initio methods. The results are compatible with the theory of quantum chemistry, but their comprovation experimental verification depend on advanced techniques for their synthesis, obtaining in laboratory for experimental biochemical.
References
- Mamikunian G, Briggs MH (Eds.). Current Aspects of Exobiology, Jet Propulsion Laboratory, California Institute of Technology. JPL technical report. 1965;32-428.
- Shaw AM. Astrochemistry: From Astronomy to Astrobiology. Wiley. 2006; 352 pages.
- Horneck G, Rettberg P. Complete Course in Astrobiology. Series: Physics Textbook, Wiley-VCH, 2007. ISBN: 3527406603, 9783527406609.
- McDouall JJW. Computational Quantum Chemistry. Molecular Structure and Properties in Silico.The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 0WF, UK, 2013.
- Patai S, Rappoport Z. The Chemistry of Organic Silicon Compounds. Series: The Chemistry of functional groups, Wiley, 1989. ISBN: 9780471914419, 047191441X, 0471919926, 0471919934, 0471623849.
- Gobato R. Infrared Spectrum for the New Nanomolecules ASi, CSi, TSi and GSi. Arch Biomed Eng & Biotechnol 5(3):2021. ABEB.MS.ID.000614.
- Gobato R, Heidari A, Valverde LF, Mitra A. Applying, "Ab Initio" Hartree-Fock Methods to Exobiological Nanomolecules. Physics of Biology. 2021;3:1-9.
- Gobato R, Heidari A, Valverde LF, Mitra A. Applying Ab Initio Hartree-Fock Methods to Exobiology Nano-Molecules. 2021;3;1-9.
- Gobato R, Heidari A, Valverde LF, Mitra A. Applying Ab Initio Hartree-Fock Methods to Exobiology Nano-Molecules. J Current Eng Technol. 3(2):134.
- Gobato R, Heidari A, Valverde LF, Mitra A. (2021) Infrared Spectrum for the New Exobiological Nanomolecules Asi, Csi, Tsi and Gsi. Sumerianz J Scientific Research. 2021;4(1):25-31.
- Gobato R, Gobato MR, Heidari A, Mitra A. Spectroscopy and Dipole Moment of the Molecule C13H20BeLi2SeSi via Quantum Chemistry Using Ab Initio, Hartree–Fock Method in the Base Set CC–pVTZ and 6–311G**(3df, 3pd)”. American J Quantum Chem Molecular Spectroscopy. 2(1)9-17.
- Pearson Education (Singapore) Pte.Ltd., Indian Branch, 482 F. I. E. Patparganj, Delhi 110 092, India, 5th ed. edition, 2003.
- Kohn W, Sham LJ. Self-consistent equations including exchange and correlation effects. Phys. Rev. (140):A1133,1965.
- Thijssen JM. Computational Physics. Cambridge University Press, Cambridge, 2001.
- Wilson AK, van Mourik T, Dunning Jr TH. Gaussian basis sets for use in Correlated Molecular Calculations. Sextuple zeta correlation consistent basis sets for boron through neon. J Mol Struct (Theochem) (388):339-349,1996.
- Polak E. Computational Methods in Optimization”, v. 77. Elsevier, 111 Fifth Avenue, New York, New York 10003, 1971.
- Gordon MS, Schmidt MW. Advances in electronic structure theory: GAMESS a decade later. Theory and Applications of Computational Chemistry: the first forty years”, Elsevier. C.E. Dykstra, G. Frenking, K. S. Kim and G.E.Scuseria (editors), pages 1167-1189, 2005.
- Gordon MS. General atomic and molecular electronic structure system (GAMESS)”. J Comput Chem. 14:1347-1363,1993.
- https://www.suse.com/products/desktop/
- https://en.wikipedia.org/wiki/Ryzen
- https://en.wikipedia.org/wiki/Asus
- https://creativecommons.org/licenses/by/4.0/
- Dennington R, Keith T, Millam J. Gaussview, Version 5, 2009.
- BIOVIA Draw 2017 Enterprise. MDL Draw Editor 17.1.0.900."Computational results obtained using software programs from Dassault Systèmes BIOVIA. The ab initio calculations were performed with the DMol3 program, and graphical displays generated with Draw." 2017.
- Brooks R, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, et al. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J Comp Chem. 1983;4:187-217.
- Brooks BR, Brooks III CL, MacKerell AD, Nilsson Jr. L, Petrella RJ, et al. CHARMM: The Biomolecular Simulation Program. J Comput Chem. 2009;30:1545-1614.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.