Reviews in Pharmacy and Pharmaceutical Sciences

Research Article - (2021) Volume 1, Issue 1
Evaluation of Acellular Dermal Matrix by Simple Method of Genomic DNA Isolation
Received Date: Oct 30, 2021 / Accepted Date: Dec 08, 2021 / Published Date: Dec 15, 2021
Abstract
The essential use of Acellular Dermal Matrix (ADM) for reconstructive surgery in deep dermal burns is well renowned. To treat the chronic wound, ADM is used as exogenous scaffold which imitate the native Extracellular Matrix (ECM) and provides a barrier to protect wounds from infection and desiccation. The dense collagen fibers and elastin of human ADM provide the strength, extensibility and elasticity which potentially reduce the scarring in deep burn patients. Although various types of synthetic as well as biosynthetic dermal matrixes available, lack of biocompatibility and absence of key factors influence the aseptic condition in unnatural way. The quality of acellular dermal matrix depends upon the methods of processing which determine the quality and that in turn affect the cellular and vascular in-growth of host. There are different types of de-cellularization methods already described in literature. Here author used the simple DNA isolation method to prove that the prepared ADM is devoid of any remaining cells and there is no genetic material. In this article, the decellularization process has been confirmed by genomic DNA isolation method.
Keywords
Dermal scaffold, Scaffold materials, Centrifugation, Cytocompatibility
Abbreviations
HADM: Human Acellular Dermal Matrix , ADM: Acellular Dermal Matrix, STSG: Split Thickness Skin Graft, IEC: Institutional Ethics committee
Introduction
A permanent Acellular Dermal Matrix (ADM) is used as typical surgical mesh which is a tissue graft generated by decellularization of human skin to save the life of deep dermal burns and improve dermal wound repair. An ideal acellular dermal matrix retains the proper biological structural integrity, biomechanical properties, cyto compatibility, porosity, structure, and surface physicochemical property [1,2]. For the treatment of full-thickness burn injury, the first Acellular Dermal Matrix (ADM) was used by Wainwright’s group and reported in 1995 [3].There are many more essential characteristic of the scaffold materials should have good biocompatibility [4]. It should have the equivalent level of biomechanical strength which provide a good adsorption interface for the adhesion surface and facilitate cell proliferation [5,6]. The scaffold should be devoid of cytotoxicity, immunogenicity and tumorigenicity in order to maintain the normal phenotype [7]. In comparison to synthetic biodegradable polymer scaffold, natural biological scaffolds have poor mechanical properties, but have good biocompatibility [8,9]. Including STSGs, various skin substitutes are used for wound coverage materials that aid in wound closure and replace the functions of the skin, either temporarily or permanently, depending on the product characteristics. In the multidisciplinary field of tissue engineering, recent developments in research yielded many novel skin replacements such as graft-jacket, primatrix, matristem, hyalomatrix, Integra, derma graft [10].Various types of scaffold materials have been reported in recent years, but these are expensive to produce. To overcome major obstacles and questing optimal skin substitute, human cadaver skin, preserved in glycerol is being used as source material of HADM. The use of acellular dermal matrix in a variety of different biomedical applications and especially for burn wound healing brings the attention of medical surgeons and research scientists to put their effort to get a biologically functional skin scaffold. Several biotechnologically derived products designed to improve dermal wound repair [11]. The acellular dermal matrix is being used for various purposes [12]. In this article author used simple method of DNA extraction method and proved the acellular matrix obtained and the properties of acellular dermal matrix did not alter the biomechanical and biological characteristics of the ADM.
Methodology
Sample collection, de-epidermalization and preparation of ADM
Sample details: For this study, cadaveric skin harvested from healthy donors who were HIV, HBsAg and HCV negative was used, as per the Institutional Ethics committee (IEC) guidelines, of National Burns Centre. Skin samples negative for the parameters were transferred into 85% glycerol containing penicillin (1 lakh unit/500 ml) and streptomycin (1 gram/500 ml). All the procedures were carried out following institutional rule. The procedure of decellularization described is similar to the cost effective decellularization of the dermis by NaOH method [13] and preparation of human ADM, processing, sterilization described previously [12].
DNA extraction and quantification
About 50 mg of reference skin and ADM were incubated for 3 to 4 hours at 54-56°C with 500 µl DNA extraction buffer (100 mMNaCl, 10 mMTrisHCl pH8, 1 mM EDTA, pH8, 1% SDS with proteinase K (20 mg/ml) until no visible material remained. Digested DED, HADM was then centrifuged at 5000 x g for 15 min to precipitate any remaining proteins. Supernatants were extracted once with phenol-chloroform-isoamyl alcohol (25:24:1) and then with chloroform-isoamyl alcohol (24:1) followed by centrifugation at 5000 x g for 15 min. Aqueous layers were removed and DNA was precipitated with ethanol, washed with 75% ethanol and finally dried. The DNA pellet was dissolved in 1 x Tris EDTA (TE).To find the action of drug, a drug is given to animals or humans to see whether the changes produced are due to the drug or by chance.
Results
Evaluation of residual genetic materials/DNA
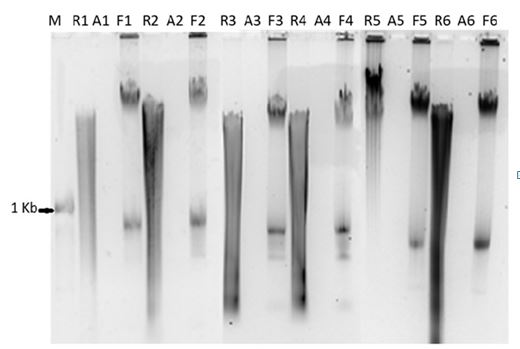
The epidermis was stripped completely after being treated with the 1 M NaCl solution. No cell component was seen in the dermis after the treatment with the 0.06 M NaOH solution. DNA was isolated from reference skin, before decellularization and the prepared ADM to evaluate the residual genetic materials of cells in the acellular dermal matrix. The amount of DNA was quantitated by measuring the optical density at 260 nm and 280 nm on a Nanodrop-1000 equipment, (Thermofisher, USA) and the calculated concentration of DNA present in the acellularized dermis and the reference skin is shown in Table 1. The total amount of DNA in reference skin is considered as 100%. From the negative values for the amount of DNA present in ADM it is apparent that there is no DNA remaining in ADM after the decellularization process. This was confirmed by gel electrophoresis where in there is no DNA seen in ADM, Figure 1. Other groups have successfully done the isolation of DNA [14] and they found very less or no DNA present. After growing the fibroblast on ADM, DNA was detected in agarose gel as shown in Figure 1 and by quantification as seen in Table 1. The clear presence of DNA in reference skin, no residual DNA in ADM and reappearance of cellular DNA seen in fibroblast growing on ADM is shown in Figure 1, in the respective wells proves that the ADM is devoid of any cells. Further the growth of skin cells (fibroblast and keratinocytes) on ADM gives the confirmation of biocompatibility of acellular dermal matrix prepared from cadaver skin.
|
S.No |
Sample ID |
Concentration (ng/ul) |
Ratio 260/280 |
Ratio 260/230 |
|
1-R |
24/16-17 |
868.9 |
1.86 |
2.07 |
|
1-A |
24/16-17 |
-56.1 |
1.19 |
0.35 |
|
1-F |
24/16-17 |
219.4 |
2.09 |
1.69 |
|
2-R |
60/16-17 |
1476.3 |
1.88 |
1.98 |
|
2-A |
60/16-17 |
-21.5 |
0.85 |
0.19 |
|
2-F |
60/16-17 |
294.6 |
2.09 |
1.6 |
|
3-R |
65/16-17 |
2061.9 |
1.84 |
2.07 |
|
3-A |
65/16-17 |
-102.5 |
1.38 |
0.56 |
|
3-F |
65/16-17 |
499.6 |
1.96 |
1.65 |
|
4-R |
70/16-17 |
898.9 |
1.81 |
2.01 |
|
4-A |
70/16-17 |
-92.3 |
1.36 |
0.59 |
|
4-F |
70/16-17 |
450.9 |
2.05 |
1.84 |
|
5-R |
71/16-17 |
827.9 |
1.78 |
1.99 |
|
5-A |
71/16-17 |
-113 |
1.41 |
0.63 |
|
5-F |
71/16-17 |
172.7 |
1.87 |
1.35 |
|
6-R |
75/16-17 |
1175.8 |
1.79 |
2.15 |
|
6-A |
75/16-17 |
-106 |
1.4 |
0.59 |
|
6-F |
75/16-17 |
152.6 |
2.89 |
1.09 |
|
R-Reference skin, A-Acellular Dermal Matrix, F-Fibroblast grown on ADM |
||||
Table 1: Evaluation of Genomic DNA: Quantification of DNA isolated from reference skin, ADM and after fibroblast grown on top of ADM. (Reference Skin (R), Acellular Dermal Matrix (ADM) and Fibroblast grown on ADM (F).
Fig.1. Agarose gel showing the DNA migrating in the wells. Reference skin DNA (R) and derived acellular matrix DNA (A) loaded adjacent to each other for the same sample ID. From the gel-pic, it is seen that there is no Cellular DNA in ADMs, thereby confirming there is no cellular component remain in ADMs. After fibroblast was grown on ADM, DNA (F) was isolated and loaded adjacent to ADM of the same sample. Further quantification of DNA (shown in Table 1), confirmed there was no DNA in ADMs and it is seen when DNA is isolated from fibroblasts are grown on ADM. (N=6).
Cytocompatibility
The ability of the ADMs to support cell growth in vitro was assessed by growing human fibroblasts at cell confluence, evaluating their cell phenotype and cell infiltration. It was noted that the fibroblasts could grow ell on the ADM. Further it was observed that the dermal facing side gives better adherence and infiltration for fibroblasts. The phase contrast image in Figure 5a clearly shows the ability of ADM to support the growth of human fibroblasts. Skin cells were grown on top of ADM and biocompatibility nature of ADM supports the cells to grow without any toxic effect.
Discussion
The most serious problem in conducting allografts is rejection. This is why physicians have used allodermisonly as a temporary covering material to treat full thickness burn wounds. This rejection is triggered by cells that show foreign MHC antigen present in both the epidermis andthe dermis [15,16].In order to mitigate a rejection response that impact tissue integration, the cellular components need to be removed [17]. It is believed that the human acellular dermal matrix will be extremely useful in a qualitative as well as cost-effective way in the management of burns and other dermal wounds. In recent decades, different classes of scaffold materials are available, these include natural biological scaffold materials, synthetic biodegradable polymer scaffolds materials [18-20] composite scaffold materials and nano scaffold materials [21-23].The growing interest of using decellularized dermal matrix and extracellular matrix (ECM)-based scaffolds holds a promising opportunity for deep dermal burns. In the field of tissue engineering and regenerative medicine, decellularized ECM is now a useful alternative in vitro model for studying the comprehensive roles of the ECM because it retains exclusively native biochemical characteristics and tissue architecture [24]. In the present study, human ADM scaffolds were made from cadaver skin and verified the biocompatibility and structural features of this material.The present work done at National Burns Centre showed that the HADM processed by non-detergent, non-enzymatic method (NaOH treatment), maintains the structural and biochemical integrity of the dermal extracellular matrix, after removal of cells of the dermis. The cell-free HADM had no residual DNA content which was confirmed by gel electrophoresis analysis. In addition to the general topographical organization of the ECM and its physical characteristics, it has been shown the growth of fibroblast on the epidermal and dermal site; however, the adherence and growth of keratinocytes were better on the epithelial side rather than onto the dermal side[25]. Author claimed that the prepared ADM is more cost effective than the commercially available material and genomic DNA isolation method is a confirmatory test for acellular dermal matrix.
Acknowledgment
The study was supported by Institutional internal project grant, National Burn Centre, Airoli, Navi Mumbai, India. The author gratefully acknowledges the complete support of Medical Director, NBC and RCBN skin bank, providing the cadaver skin for the study. Author offers thanks to all the medical and non-medical staff of the National Burns Centre.
Conflict of Interest
The author has declared no conflict of interest.
References
- Zhu C, Ying D, Mi J, et al. Development of anti-atherosclerotic tissue-engineered blood vessel by A20-regulated endothelial progenitor cells seeding decellularized vascular matrix. Biomaterials. 2008; 29:2628-2636.
- Liu X, Wang J, Dong F, et al. Study of composite vascular scaffold combining with differentiated VSMC- and VEC-like cells in vitro and in vivo. J Biomater Appl. 2017; 32:219-229.
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995; 21:243-248.
- Wang L, Yang J, Ran B, et al. Small molecular TGF β1 inhibitor loaded electrospun fibrous scaffolds for preventing hypertrophic scars. ACS Appl Mater Interfaces. 2017; 9:32545-32553.
- Faulk DM, Londono R, Wolf MT, et al. ECM hydrogel coating mitigates the chronic inflammatory response to polypro-pylene mesh. Biomaterials. 2014; 35:8585-8595.
- Wang W, Deng D, Wang B, et al. Comparison of autologous, allogeneic, and cell free scaffold approaches for engineered tendon repair in a rabbit model a pilot study. Tissue Eng Part A. 2017; 23:750-761,
- Ramanathan G, Muthukumar T, Sivagnanam U. In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro inflammatory cytokines in wound healing. Eur J Pharmacol. 2017; 814:45-55.
- Awad NK, Niu H, Ali U, et al. Electrospun fibrous scaffolds for small diameter blood vessels: A review. Membranes (Basel). 2018; 8:E15.
- Xu Y, Wu J, Wang H, et al. Fabrication of electrospun poly (L lactide co-ε-caprolactone)/collagen nanoyarn network as a novel, three dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng Part C Methods. 2013; 19:925-936.
- Nicholas MN, Yeung J. Current status and future of skin substitutes for chronic wound healing. J Cutan Med Surg. 2017; 21(1):23-30.
- Juhasz I, Kiss B, Lukacs L, et al. Long-term follow up of dermal substitution with acellular dermal implant in burns and post burn scar corrections. Dermatol Res Pract. 2010; 2010:210150.
- Rossner E, Smith MD, Petschke B, et al. Epiflex (®) a new decellularised human skin tissue transplant: Manufacture and properties. Cell Tissue Bank. 2011;12(3):209-217.
- Richters CD, Pirayesh A, Hoeksema H, et al. Development of a dermal matrix from glycerol preserved allogeneic skin. Cell Tissue Bank. 2008; 9(4):309-315.
- Hogg P, Rooney P, Ingham E, et al. Development of a de-cellularized dermis. Cell Tissue Bank. 2013;14(3):465-474.
- Takami Y, Matsuda T, Yoshitake M, et al. Dispase/detergent treated dermal matrix as a dermal substitute. Burns. 1996; 22(3):182-90
- Badylak SF, Matheny R, Obermiller J, et al. ECM scaffold for myocardial reconstruction. Symposium on tissue engineering science. Aegean Conferences Series. 2002; 4:65.
- Xu H, Wan H, Sandor M, et al. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A. 2008; 14(12):2009-2019.
- Farkas B, Rodio M, Romano I, et al. Fabrication of hybrid nanocomposite scaffolds by incorporating ligand free hydroxyapatite nanoparticles into biodegradable polymer scaffolds and release studies. Beilstein J Nanotechnol. 2015; 6:2217-2223.
- Nasonova MV, Glushkova TV, Borisov VV, et al. Biocompatibility and structural features of biodegradable polymer scaffolds. Bull Exp Biol Med. 2015; 160:134-140.
- Perry L, Flugelman MY, Levenberg S. Elderly patient derived endothelial cells for vascularization of engineered muscle. Mol Ther. 2017; 25:935-948.
- Hu WW, Wu YC, et al. The development of an alginate/polycaprolactone composite scaffold for in situ transfection application. Carbohydr Polym. 2018; 183:29-36.
- Sun T, Liu M, Yao S, et al. Biomimetic composite scaffold containing small intestinal submucosa and mesoporous bioactive glass exhibits high osteogenic and angiogenic capacity. Tissue Eng Part A. 2018; 24:1044-1056.
- Tohamy KM, Mabrouk M, Soliman IE, et al. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int J Biol Macromol. 2018; 112:448-460.
- Hoshiba T, Chen G, Endo C, et al. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016; 2016:6397820.
- Xiao S, Zhu S, Ma B, et al. A new system for cultivation of human keratinocytes on acellular dermal matrix substitute with the use of human fibroblast feeder layer. Cells Tiss Org. 2008; 187:123-130.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.