Reviews in Pharmacy and Pharmaceutical Sciences

Research Article - (2021) Volume 1, Issue 1
Formulation and Biological Implication of Eucalyptus Globulus and Trachspermum ammi Organogel for Topical Delivery
2Deptartment of Chemistry and Pharmacy, Rani Durgavati University, Madhya Pradesh, India
Published Date: Jan 03, 2022
Abstract
Topical delivery is an interesting option because it is convenient and safe. This offers several potential advantages over conventional routes like avoidance of first-pass metabolism, predictable, minimizing undesirable side effects and most importantly, it provides patient compliance as the drug delivery is painless. Eucalyptol is a hydrophobic, anti-inflammatory drug with a shorter biological half-life. In this context, the jellified emulsion was formulated using Na CMC as a polymer, liquid paraffin as oil phase, emulsifying agents like span 80 and tween 80, oleic acid, and clove oil as permeation enhancers. Studies were carried out with the aim to develop organogel with the different gelling agents for topical application and evaluation of these formulations.
Keywords
First pass metabolism, Hydrophobic, Gellified emulsion, Permeation enhancers
Introduction
Topical drug delivery system
In Topical drug delivery system the medication is distributed throughout the body through the systemic blood circulation. Topical drug administration is anywhere in the body through ophthalmic, rectal, vaginal and dermal as topical routes. Skin is one of the important routes and widely distributed accessible organ on human body for topical administration found to be major route in topical drug delivery system. Skin Route is recognized as an effective means of therapeutic and local dermatological diseases [1]. Topical utilization of medications offers potential points of interest of conveying the medication straightforwardly to the site of activity and representing an amplified timeframe [2,3]. Topical planning maintains a strategic distance from the GI-irritation, avoid the metabolism of medication in the liver and increase the bioavailability of the medication. In the form of topical dosage form, endeavors are being made to use sedate bearers that satisfactory restriction or infiltration of the medication inside or through the skin in order to enhance the local and minimize the systemic impact, or to ensure sufficient absorption [4-6].
Advantages of topical drug delivery systems
- Avoid the first pass metabolism.
- Conveniently applicable and easy to use.
- Avoid the dangers and burdens of intravenous treatment and the fluctuated states of assimilation, similar to pH changes, nearness of catalysts, gastric purging time and so on.
- Ability to effectively end the medications, when required.
- Avoids fluctuation in medication levels, between inter and intra-patient varieties.
- A moderately large area of application in correlation with buccal or nasal cavity.
- Capacity to deliver drug more specifically to a particular site.
- Avoidance of gastro-intestinal incompatibility.
- Providing use of medications with short biological half-life, narrow therapeutic window.
- Improve patient compliance.
Principles of topical permeation
Before a topically applied drug can act either locally or systemically, it must penetrate the stratum corneum, the skin permeation barrier. Percutaneous absorption involves passive diffusion of substances through the skin. The mechanism of permeation can involve passage through the epidermis itself (trans-epidermal absorption) or diffusion through shunts, particularly those offered by the relatively widely distributed hair follicles and sweat glands (trans-follicular or shunt pathway). In the initial transient diffusion stage, the drug molecules may penetrate the skin along the hair follicles or sweat ducts and then absorbed through follicular epithelium and the sebaceous glands. When steady state has been reached the diffusion through the intact stratum corneum becomes the primary pathway for the topical permeation. The release of a therapeutic agent from a formulation applied to the skin surface and its transport to the systemic circulation is a multistep process.
Types of organogels
Lecithin organogels: Lecithin organogels have emerged as one of the most potential carrier systems. The organogel matrix mainly consists of a surfactant (lecithin) as gelator molecules, a nonpolar organic solvent as external or continuous phase, and a polar agent, usually water [7-9]. A lecithin organogel is formed when small amounts of water or other polar substances, such as glycerol, ethylene glycol or formamide, are added to a non-aqueous solution of lecithin. The transfer into jelly-like state has been demonstrated only for non-aqueous solutions of naturally occurring unsaturated lecithin. The latter are mainly separated from soy bean and egg yolk
(Figure1).
Fig.1. Preperation and formulation of various types of organogel.
Sorbitanmono stearate organogels: Made up of combination of Sorbitanmono stearate (Span 60) and sorbitanmono palmitate (Span40) have been found to gel a number of organic solvents at low concentrations.
Nano-emulsion based organogels: Microemulsions are defined as thermodynamically stable transparent, single optically isotropic liquid system of water, oil and surfactants frequently in combination with suitable co-surfactants. Microemulsions are known to enhance the bioavailability of drugs via topical and systemic routes [10].
Poly (ethylene) organogels: Very few polymeric organogels have been geared towards pharmaceutical applications [11-17]. The only two such systems have been widely tested for drug delivery applications are poly (ethylene) organogels. Poly (ethylene) organogels patches were shown to be non-irritating and have low sensitizing properties.
Supramolecular organogels: Although a low molecular mass gelator was discovered in the early nineteenth century, the supramolecular nature of these materials was poorly understood and they were largely neglected until the late 20th century. The diversity of gel structural architectures has allowed them to be utilized as templates to prepare novel inorganic superstructures for possible applications in catalysis and separation [18-22].
Eudragit organogels: Eudragitorgano gels are really mixtures of Eudragit (L or S) and polyhydric alcohols, such as glycerol, propylene glycol and liquid polyethylene glycol containing high concentrations (30 or 40% w/w) of Eudragit. Gel consistency and spreading is described using a penetrometer. Gel viscosities were found to increase with increasing concentrations of Eudragit and to decrease with increasing drug content [23-28].
Methodology
Materials, chemicals and reagents
The following materials used in the study are Pharma grade or the best possible Laboratory Reagent (LR) supplied by the manufacturer (Table 1).
|
Sl. No. |
Materials |
Source |
|
1 |
Eucalyptol |
Yarrow chemicals, Mumbai |
|
2 |
Thymol |
Yarrow chemicals, Mumbai |
|
3 |
Sodium Alginate |
Otto chemical reagents |
|
4 |
Guar gum |
Yarrow chemicals, Mumbai |
|
5 |
Xanthan gum |
Merck ltd |
|
6 |
Methyl paraben |
Nice Chemicals Pvt. Ltd., Kerala |
|
7 |
Pluronic F127 |
Yarrow chemicals, Mumbai |
|
8 |
Methanol |
Karnataka fine chem., Bangalore |
Table1. List of chemicals and reagents.
Preparation of eucalyptol and thymol organogels
For the preparation of eucalyptol and thymol organogels, the organogel composition of different formulations of eucalyptol and thymol organogel F1-F6 and organogel composition of different formulations of eucalyptol and thymol organogel F1-F6 are shown in Tables 2 and 3 [29].
|
Ingredients (mg) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Eucalyptol (ml) |
12 |
12 |
12 |
12 |
12 |
12 |
|
Thymol |
40 |
60 |
80 |
40 |
60 |
80 |
|
Sodium alginate |
20 |
40 |
60 |
- |
- |
- |
|
Guar gum |
- |
- |
- |
20 |
40 |
60 |
|
Xanthan gum |
- |
- |
- |
- |
- |
- |
|
Pluronic F-127 |
40 |
40 |
40 |
40 |
40 |
40 |
|
Methyl paraben |
30 |
30 |
30 |
30 |
30 |
30 |
|
Methanol (ml) |
10 |
10 |
10 |
10 |
10 |
10 |
|
Water |
QS |
QS |
QS |
QS |
QS |
QS |
Table 2. Organogel composition of different formulations of eucalyptol and thymol organogel F1-F6.
|
Ingredients (mg) |
F7 |
F8 |
F9 |
F10 |
F11 |
F12 |
|
Eucalyptol (ml) |
12 |
12 |
12 |
12 |
12 |
12 |
|
Thymol |
40 |
60 |
80 |
40 |
60 |
80 |
|
Sodium alginate |
- |
- |
- |
25 |
40 |
55 |
|
Guar gum |
- |
- |
- |
30 |
45 |
60 |
|
Xanthan gum |
20 |
40 |
60 |
35 |
50 |
70 |
|
Pluronic F127 |
40 |
40 |
40 |
40 |
40 |
40 |
|
Methyl paraben |
30 |
30 |
30 |
30 |
30 |
30 |
|
Methanol |
10 |
10 |
10 |
10 |
10 |
10 |
|
Water |
QS |
QS |
QS |
QS |
QS |
QS |
Table 3. Organogel composition of different formulations of eucalyptol and thymol tablets F7-F12.
Method of preparation of organogel
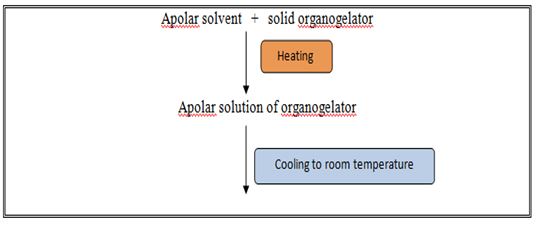
Organo gelators precipitates out as fibers which undergo, physical interactions amongst each other thereby forming, a 3-dimentional networked structure, which immobilizes the polar solvent (Figure 2).
Fig. 2. Method of formation of organogel by solid fiber mechanism.
Discussion
Biological properties
Insecticidal: Plant secondary metabolites play an important role in the plant-insect interactions. Some compounds extracted from plants have insecticidal activity. Essential oil extracted from the seeds of ajwain exhibited insecticidal activity against Callosobruchus chinensis in the ovi-position step as well as egg hatching and developmental inhibitory activities [31].
Antibacterial: Ethanol and acetone extract of ajwain seeds possessed an antibacterial activity against two Gram negative food spoilage bacteria Pseudomonas aeruginosa and Escherichia coli. The in vitro antibacterial activity was performed by disc diffusion method. Ethanol extract of ajwain seeds possessed highest activity against P. aeruginosa whereas acetone extract exhibited highest activity against E. coli. Antibacterial effect of ajwain was studied by applying cream containing 5% essential oil of ajwain for healing wound in rabbits and compared its effect with iodine solution [32]. Wound contraction on the 15th day in ajwain group was 99.68%, compared with the healing effect of iodine solution group and non-treatment group which was found to be 100 and 96.57%, respectively which indicated a wound healing effect of ajwain.
Antifungal: Ethanol extract of ajwain seeds showed antifungal activity against selected fungi (Aspergillus flavus, A. ochraceus, A. niger, A. orzyae, Fusarium moniliforme, Penicillium sp.) using agar well diffusion assay. Ajwain seed essential oil also exhibited a broad spectrum of fungitoxic behavior against A. niger, A. flavus, A. oryzae, A. ochraceus, F. monoliforme, F. graminearum, P. citrium, P. viridicatum, P. madriti and Cheilomenes lunata and absolute mycelial zone inhibition was obtained at a 6 µl dose of the oil.
Anthelmintic: Anthelmintic activity in ajwain was exerted by interference with the energy metabolism of parasites through potentiation of ATPase activity and thus loss of energy occured. Anthelmintic activity of ajwain, showed its effect against specific helminths, e.g. Ascaris lumbricoides in humans and Haemonchus contortus in sheep. The plant was also reported to possess cholinergic activity with peristaltic movements of the gut, thus helped in expulsion of intestinal parasites which might also be a contributory factor to its anthelmintic activity [33].
Conclusion
Eucalyptol and thymol are volatile oil is a hydrophobic in nature, anti-inflammatory agent with shorter biological half-life. It has GIT, renal and hepatic disorder if taken orally. Hence, to overcome the above drawback it is required to administer the drug topically. The pre-formulation studies involving solubility and melting point of the drug were found to be comparable with the standard. The drug eucalyptol and thymol was checked for compatibility with selected polymers by FTIR and found to be compatible. Formulation of organogel was carried out using Sodium Alginate, Guar Gum and Xanthan gum as gelling agent. The prepared topical organogel of Eucalyptol and thymol were formulated and subjected to physicochemical studies i.e. viscosity, spreadability studies and in vitro release studies. Drug content of all the formulations were found to be in the range of 86-103 % and pH was found to be between 6-7 for all the formulations. All the formulations showed good spreadability.
The stability study as per ICH guidelines was performed for the optimized formulation. F6 formulation at Accelerated testing of 40°C ± 2°C/70 ± 5% RH. No major change in appearance, pH and drug content was seen. Finally, it could be concluded that the prepared organogel formulation was found to be an effective vehicle for the delivery of the drug and showing the biological properties i.e. insecticidal, antifungal, antibacterial and anthelmintic. Further studies should be carried out to prove the clinical effectiveness of the formulation.
Acknowledgement
Author Mohd. Washid Khan thanks Prof. Kapil deo Mishra, Vice Chancellor, Rani Durgavati University, Jabalpur for providing medium-sized libraries and workplaces. I would also like to inform Dr. Brajesh Singh, Registrar, Rani Durgavati University, Jabalpur Instrumentation Provisioning and Laboratory Facility.
Conflict of Interest
The authors declare no conflict of interest
References
- Mikari BV, Mahadik KR. Formulation and evaluation of topical liposomal gel for fluconazole. Indian J Pharm Sci. 2010; 44(4):324-325.
- Glavas-Dodov D. 5-Flurouracil in topical liposome gels for anticancertreatment formulation and evaluation. Act a pharm. 2003; 53(4):241-250.
- Kumar NPM, Patel MR, Patel KR, et al. Emulgels: a novel approach to topical drug delivery. Int. J of Uni Pharm Bio Sci. 2013; 2(1):134-148.
- Debnath S, Vanitha G, Bindu HP, et al. Applications of organogels in drug delivery. Indian J Res Pharm Biotech. 2014; 2(1):976-981.
- Mujawar NK. Organogel: Factors and its importance. Int. J of pharm Chem Biol Sci. 2014; 4 (3):758-773.
- Ross and willson anatomy and physiology 11th edn. p.34-38.
- Goyal S, Sharma P, Ramchandani U, et al. Novel anti-inflammatory topical gels. Int J of Pharm Biol Arch. 2011; 2(4):1087-1094.
- Bharadwaj S, Gupta GD, Sharma VK. Topical Gel: A Novel approach fordrug delivery. J Chem Biol Phys Sci. 2012; 2(2):856-867.
- Chien YW. Transdermal therapeutic system, Marcel Dekker, Inc., Madison Avenue, New York, 2005; 2(29):528-531.
- Jain NK, Mishra AN. Controlled and novel drug delivery. New Delhi. 2005:101-106.
- Neha JR, Puja JH, Javesh PK. Formulation and evaluation of topical antibacterial herbal gel of coriander and eucalyptus oil. World J Pharm and Pharmaceutical sci. 2014; 3(7):774-786.
- Kaur LP, Guleri TK. Topical gel: A recent approachfor novel drug delivery. Asian J Biomed Pharm Sci. 2013; 3(17):1-5.
- Patil K, Bakliwal S, Pawar S. Organo gel: Topical and transdermal drug delivery system. Int J Pharm Res Dev. 2011; 3(6):58-66.
- Reddy CG, Reddy AB, Jotish M, et al. Organo gels- A review. Int J Pharm Technol. 2010; 2(4):584-602.
- Pawar SA, Patil MP. Review on organogel as topical delivery system. World J Pharm Sci. 2014; 3(10):393-409.
- Debnath S, Vanitha G, HimaBindu P, et al. Applications of organogels in drug delivery. Indian J Res Pharm Biotech. 2014; 2(1):976-981.
- Mohamed MI. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004; 6(3):1-7.
- Sabu KR, Basarkar GD, Formulation, development and in-vitroevaluation of terbinafine hydrochloride emulgel for topical fungal infection. Int J Pharm Pharmaceut Sci. 2013; 21(2):168-173.
- Deveda P, Vyas N, Khambete H, et al. Gellified emulsion for sustain delivery of Itraconazole for topical fungaldisease. Int J Pharma Pharmaceutical Sci. 2010; 2(1):104-112.
- Baibhav J, Gurpreet S, Rana AC, et al. Development and characterization of clarithromycin emulgel for topical delivery. Int J Drug Dev Res. 2012;4(3):310- 323.
- Khunt DM, Mishra AD, Shah DR. Formulation design and development of piroxicam emulgel. Int J PharmTech Res. 2012; 4(3):1332-1334.
- 22.Priya RM, Sellakumar V, Natarajan R, et al. Formulation and in-vitro evaluation of ciprofloxacin loaded topical emulgel. Int J pharm Chem Sci. 2012;1(1):237-242.
- Khullar R, Kumar D, Seth N, et al, Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharmac J. 2012; 20(1):63-67.
- Uplanchiwar RV, Bhadoria S , Gahane A, et al. Comparative evaluation of Zidovudine loaded hydrogels and emulgel Research J Pharm Tech. 2011;5(1):41-43.
- Jain A, Gautam SP, Jain GS. Development and characterization of Ketoconazole emulgel for topical drug delivery. Der Pharmacia Sinica. 2010; 1(3):221-231.
- Hosny KM, Rambo SM, Al-Zahrani MMet al. Ketoprofen emulgel: Preparation, characterization, and pharmacodynamic evaluation. Int J Pharm Sci Rev Res. 2013; 20(2): 306-310.
- Chaubey MK. Fumigant toxicity of essential oils from some common spices against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). J Oleo Science. 2008; 57(3):171-179.
- Kostyukovsky M, Rafaeli A, Gileadi C, et al. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag Sci. 2002; 58(11):1101-1106.
- Masih U, Shrimali R, Naqvi SMA. Antibacterial activity of acetone and ethanol extracts of cinnamon (Cinnamomum zeylanicum) and ajwain (Trachyspermum ammi) on four food spoilage bacteria. Int Res J Biol Sci. 2012; 1(4):7-11
- Gilani AH, Jabeen Q, Ghayur MN, et al. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethno pharmacol. 2005; 98(12):127-135.
- Thangam C, Dhananjayan R. Anti-inflammatory potential of the seeds of Carum copticum Linn. Indian J Pharmacol. 2003; 35:388-391.
- Jabbar A, Iqbal Z, Khan MN. In vitro anthelmintic activity of Trachyspermum ammi seeds. Pharmacogonosy Magazine. 2006; 2:126-129.
- Tamurab T, Thymol IH. A classical small molecule compound that has a dual effect (potentiating and inhibitory) on myosin. Biochem Biophys Res Comm. 2004; 18: 86-789.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.