Reviews in Pharmacy and Pharmaceutical Sciences

Mini Review - (2022) Volume 2, Issue 1
Scope of Organometallic Compound and their Profound Biological Implication - A Review
2Department of Jagmohan Das Ward, Silicobyte Katni Degree College, Madhya Pradesh, India
Received Date: Jan 27, 2022 / Accepted Date: Feb 21, 2022 / Published Date: Feb 28, 2022
Abstract
The combination of steel provides opportunities to develop structures with well-defined structures. In which most of contagious diseases and their prevention will be depend on the metabolism of organic and nonorganic elements. Blending chemistry offers interconnectivities to use metal detectors as secret agents. These integration combinations show significant variability in performance. Metal ions and these bonds can improve the function of active moieties. This paper updates about diversity the use of metal structures in the physiological and biological system. This review summarizes the latest advances in organometallic problems for the use of diagnostic and therapeutic applications in detail with advances focused on organometallic technology.
Keywords
Inorganic metal complexes, Biological activity, Metal complex ect
Introduction
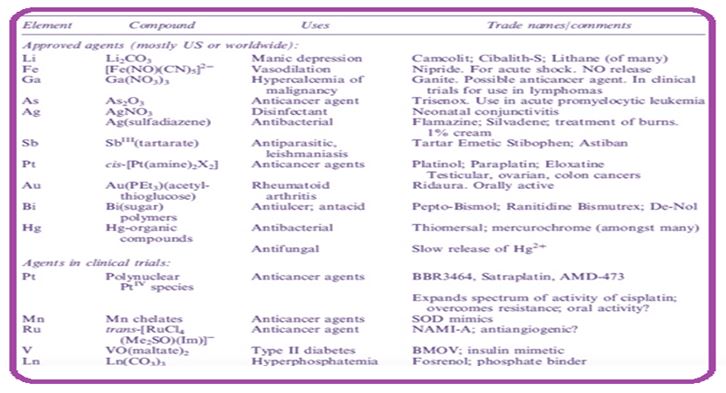
Inorganic medical chemistry lies in the interaction of medicine with inorganic chemistry, and includes iron-based drugs, metal detection and binding agents and iron containing diagnostic resources [1-4]. In the first systematic study of iron in medicine (mid-first to mid-twentieth century), recognition of the importance of other iron ions (e.g. iron, zinc and copper) in order to avoid deficiency was a major step forward. Not only others, many iron ions are important elements, but many are also increasingly being developed as diagnostic or therapeutic components for the study or treatment of various diseases and metabolic disorders [4,5]. The list of metal ions suitable for the critical situation is an ongoing process; it included not only the expected compounds such as zinc, copper and manganese but also many who thought of just toxins, such as selenium and molybdenum, listed as "potentially important" unexpected people such as arsenic, nickel, silicon, and vanadium [6-9]. Organo-metallic compounds can be used as a miracle drug for various diseases for period of hundred years. Metallic structures play a vital role in medicine and agriculture. Metal elements play a kinetic role at the cellular level in the life process [10].
Schiff Bases metal Complexes
The link between diabetes and iron imbalance makes iron-based treatment a fascinating proposition. The progression of anti-diabetic structures that replace insulin injections to control blood sugar moiety seems exciting. It can be observed that the regulation of glucose levels in blood fluid has been play by the regulation of vanadium and zinc in the form of inorganic salts. The amount of vanadium content and other metallic component has been formed and all have play insulin-mimetic properties [11]. Antiviral material compared to the Schiff base. The structure of pyridone, pyrolidone and o-phenylenediamine with relevant complex have excellent anti-bacterial activity. The Schiff base containing metals such as Arsenic, Antimony and Bismuth exhibits antifungal properties against A. niger and A. alternata. The schiff compound and their relevant structures built between furan or amyl furylglycoxal show anti-fungal activity against biodiversity. Silver structures in the state of oxidation have shown inhibition against cucumber mosaic virus [12]. The iron properties of Furan semi carbazone show important antihelmintic and analgesics activities [13]. Schiff base metal structures have antifertility and enzymatic activity and chromium azomethine complexes, the cobalt complex of Schiff foundations are used in skin dye, food package [14,15]. Antibiotic-resistant diseases in the health care system and the population have put a strain on the economy and adequate health care. There is an urgent need to develop new synthetic components with a better and more acceptable therapeutic index [16].
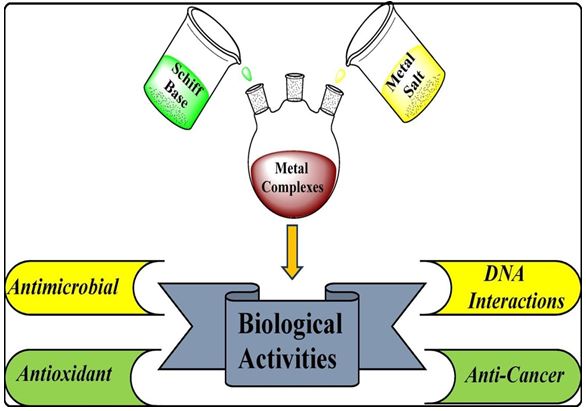
Fig.1. Diagrammatic representation of biological activities of metal complex.
OrganometallicCompounds
Organometallic compounds are often classified as “organo-,” as in organo palladium component. They are also called as organo-inorganics, metallo-organics, and metalorganics. Certain examples of metal organic compounds include all Gilman reagents containing lithium and copper, and grignard containing regenerative magnesium. Tetracarbonyl nickel and ferrocene are examples of organometallic compounds that contain flexible metals [17]. Metal organic compounds are widely used both stoichiometric in research and industrial chemical reactions, as well as in the role of activators to increase such reaction levels, and in the use of homogeneous catalysis, where targeted compound comprise polymers, pharmaceuticals, and many other forms of catalysis. The Metal complex consists of a medium (or) metal atom bound to anion called ligands.
Fig.2. 3D molecular structure showing the organometallic compound.
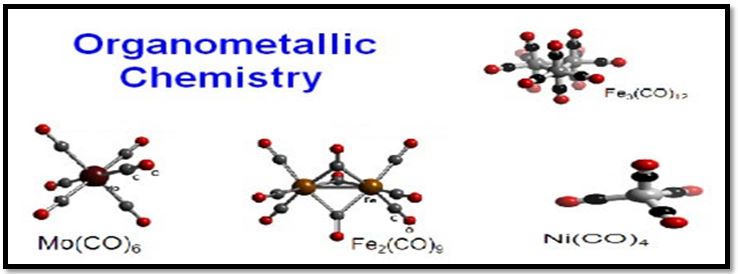
Fig.3. Some of the recent example of organometallic compound.
Metal Complexes in Biological system
Metal ions form bonds and ligands through the process, to release and slow down biological processes. An essential element present in the biological system is the metal that plays a vital role in all biological system. Several metals are important in the chemical composition of living things, the most common examples being iron, cobalt, copper, and molybdenum. Iron is a widely used and important transforming tool that works in living systems; iron-containing proteins participate in two main processes, the transport of oxygen and the transfer of electrons (i.e., reduced oxidation). There are also many things to do with storing and transporting the iron itself. There are a number of occasional improvements depending on the facile synthesis and potential applications. Like drugs used as living molecules and their metals, metal structures have been found to improve due to increased lipophilic properties compared to the corresponding free ligand molecules [18].
Fig.4. Therapeutic and prospective medical uses of inorganic compounds.
Metal Complex as Platform in Cancer Therapy
In medical chemistry, therapeutic use of metal structures in cancer and leukemia has been reported since the sixteenth century. Today it is still one of the best-selling anti-cancer drugs. Complexes composed of other metals such as Cu, Au, Ga, Ge, Sn, Ru, Ru, Rh, Ir were shown the important antitumor activity in animals. Some latest developments provide new targets for anti-cancer agents that build up DNA compounds and cancer cells and cause blockade of DNA multiplication. But it also damage normal cells like hair follicles, mucous membranes in the intestines and so on. The drugs are combined with a porphyrin ring to improve the specificity of complex tumor tissue [19]. In this view, we want to provide a review of previous updates on the cytotoxic effect of metal-based structures while focusing on the recent develop metal complex problems made of steel and their cytotoxic effects on cancerous cell, as well as the new metallic moiety used in drug-based drug development and targeted treatment for carcinoma [20].
Metal Chelators for their Potential used in Neurological Disorder
The formation of a non-toxic active agent for complex and complete mental disorders represents a very challenging task. There is much to understand about the etio pathogenesis of AD, PD and prion disease. However, chelate formation may be considered an important strategy for both treatment and neuro degeneration research. In the treatment of various neurological diseases iron also plays an important role such as Lithium complex with drug molecules can cure many neurological diseases such as Huntington's chorea, Parkinsonism, live brain disorders, epilepsy and paralysis etc. The neutral phase represented by Isonicotinoyl Picolinoyl Hydrazine (IPH) may appear promising. These molecules bind iron and two atoms of nitrogen and oxygen, thus binding iron (III), copper (II), and zinc (II) together. A hydrophobic phenathroline analogue, bantucuproine has been shown to help resolve Aβ from AD brain samples [21]. Numerous multidisciplinary and multidisciplinary studies in the treatment of metal chelation highlight the need to improve computer integration, testing, and analysis in order to successfully introduce anti-AD chelating drugs. Many drug students have limitations in their physicochemical features; some promote the redistribution of iron ions, while others activate the signaling mechanisms by AD [22].
Metal Complexes in the Management of Diabetes
The development of anti-diabetic structures that replace insulin injections to control blood sugar levels seems exciting. It has been observed that the regulation of glucose levels in blood plasma has been achieved by the regulation of vanadium and zinc in the form of inorganic salts. The amount of vanadium and other metals is made and they all show insulin-mimetic properties. This paper focuses on the major role of metals and their properties in biological systems and their therapeutic uses. Diabetes mellitus is an incurable disease characterized by hyperglycemia, which means an increase in blood glucose. Hyperglycemia is caused by insulin deficiency. In diabetes mellitus, intake of chromium metal complex has shown a significant decrease in glucose level 6. New insulin mimetic zinc (II) properties have been found to have different binding properties and have a lowering of blood sugar levels to treat type 2 diabetes in animals.
Conclusion
Compounds provide a platform for designing and develop therapeutical components. The basic ideas for integrating and elaborating various processes in the metallic complex are ongoing. Although it has many side effects, it is still widely used in the advancement of cancerous cell line. The formation of a non-toxic active agent complex can be treated mental disorders represents a very challenging task. Despite the success of current complex metal treatments, there are some drawbacks. There is therefore a need for new ways needed to avoid these pitfalls.
Acknowledgment
Author Mohd.Washid Khan thanks Prof. Kapil deo Mishra, Vice Chancellor, Rani Durgavati University, Jabalpur for providing medium-sized libraries and workplaces. I would also like to inform Dr. Brajesh Singh, Registrar, Rani Durgavati University, Jabalpur Instrumentation Provisioning and Laboratory Facility.
Conflict of Interest
The authors declare no conflict of interest.
References
- Knoll JD, Turro C. Control and utilization of ruthenium and rhodium metal complex excited states for photo activated cancer therapy. Elsevier. 2015; 282-283:110-126.
- Jamieson ER, Lippard SJ. Structure, recognition and processing of cisplatin DNA adducts. Chem. Rev. 1999 1-5.
- Piulats JM, Jimenz L, Villanuera A, et al. Molecular mechanics behind the resistance of cisplatin in germ cell tumours. Clin Transl Onco. 2009; 12:780-786.
- Loo C, Lin A, Hirsch L, et al. Nanoshell enabled photonics based imaging and therapy of cancer. Technol cancer Res Treat. 2004; 3:33-40.
- Hashimoto R, Fujimak K, Jeong MR, et al. Neuroprotective action of lithium. Seishin shinkeigatu zasshi. 2003; 105:81-86.
- Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids. A systemic review of randomized controlled trials. Diabetes care. 2007: 30:2154-2163.
- Hiromusakurai and Yusuke Adachi. The pharmacology of the insulinomimetic effect of zinc complexes. Biometals. 2006; 18:319-323.
- Gaur S. Phsicochemical and biological properties of Mn(II), Ni(II) and chelates of Schiff bases, Asian J Chem. 2003; 15:250-254.
- Dhakrey R, Saxena G. Synthesis of Ni (II) complexes with heterocyclic aldehyde Schiff base. Ind Chem J Soc. 1987; 64:685-686.
- Agharwal S. Drug-metal complexes in research-A review. IJSMER. 2016; 1:10-11.
- Saba M, Tejaswi B, Chitra K, et al. Metal complexes in the management of diabetes mellitus: A new therapeutic strategy. Int J Pharm Pharm Sci. 2020; 6:40-44.
- Meng F, Zhao Q, Xin LY. Yingyong Hu axue. 2002; 19:1183-1185.
- Latha KP, Vaidya VP, Keshavayya J. Synthesis, characterization and biological investigations on metal complexes of 2- acetyl naptha (2,1-b) furan semicarbazone. J Teach Res Chem. 2004; 11:39-48.
- Sing R, Gupta N, Fahmi N. Biochemical aspects of dioxomolybdenum (VI) and manganese (II) complexes. Indian J Chem. 1999; 38:1150-1158.
- Befta. Unsymmetrical 1:2 chromium complex dyes. Fabio (to Ciba Geigy AG), Eur Pat Abstr. 1985; 103:161871.
- Priyanka G, Anita C, Dinesh K, et al. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorg Chem Comm. 2021; 130:1-5.
- Crabtree RH. The organometallic chemistry of the transition metals. Hoboken, NJ: Wiley. 2005; 2-5.
- Ezzat K, Muhammad H, Muhammad SA. Schiff bases and their metal complexes with biologically compatible metal ions; biological importance, recent trends and future hopes. Rev Inorg Chem. 2021; 10:34.
- Agharwal S, Drug-metal complexes in research-A review. IJSMER. 2016; 1:10-11.
- Martínez MM, Carranza P, Massaguer A, et al. Synthesis and biological evaluation of Ru(II) and Pt(II) complexes bearing carboxyl groups as potential anticancer targeted drugs. Inorg Chem. 2017; 56:13679-13696.
- Alessandra G, Robert CH. The crucial role of metal ions in neurodegeneration: The basis for a promising therapeutic strategy, Br J Pharmacol. 2005; 146:1041-1059.
- Fasae KD, Abolaji AO, Faloye TR, et al. Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer’s disease: Limitations and current and future perspectives. 2021; 67:126779.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.